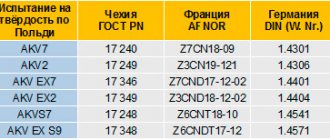
Electrolytic polishing
You can place an order for electropolishing of products by filling out the form below or by referring to our article in the “Services” section: Electropolishing service.
Electrolytic polishing is a process used to polish a metal surface using an electric current and a chemical solution, using a container equipped with electrodes. This process produces a mirror-like surface by selectively removing the surface from the steel.
This selective removal is carried out by controlled electric current and special electrolyte solutions. Electrical parameters are adjusted using the INVERTER technology built into our CLINOX products, while an electrolytic solution called E-polishing Bomar is used with our E-polishing Box, made from acid-resistant plastic and carbon electrodes. fibers to ensure better performance and complete safety.
Thanks to this combination of products the following results can be obtained
Chemical method
Small stainless steel parts are processed using a method that does not require much physical effort and several hours of work. Using circles can just be awkward. Immerse the cleaned workpiece in a bath with strictly dosed reagents, diluted to the required concentration with distilled water. Over a sufficient period of time, under the influence of caustic reagents, all steel roughness in contact with the liquid active medium is eliminated. Deep scratches and welding marks are first leveled with emery wheels, then smoothed with soft circles with paste of the required grain size (GOI). Otherwise, all large flaws will also be polished while maintaining their shape.
To correctly select components and their concentration in the water mass, it is advisable to know the grade of stainless steel:
- Brand X18N9T is immersed in the following composition: acids: 230 ml sulfuric, 40 ml nitric, 70 ml hydrochloric. To 1 liter of solution add acid black dye - 6 g, wood glue - 10 g, sodium chloride - 6 g. The liquid temperature is maintained at 65-70 ° C, time 5÷30 minutes.
- Acids in proportion to the total volume: nitric 4÷5%, orthophosphoric 20÷30%, hydrochloric 3÷4%, methyl orange - 1÷1.5%, in an aqueous solution with a temperature of 18÷25 ° C, Approximate holding time 5÷ 10 min .
- Per liter of composition the amount of acids: sulfuric 230 g, hydrochloric 660 g, orange acid dye - 25 g. Maintain a temperature of 70÷75 ° C, time 2÷3 minutes.
To complete the reaction at all points and remove the resulting products, the liquid in the container is continuously stirred. You can move the steel part.
The components are aggressive. Provide protection for the skin of the hands, face, eyes, and respiratory organs.
Chemical alignment of the line of the outer boundary of the stainless steel (polishing) occurs because the reaction is more intense on the protrusions of the profile. To prevent the accumulation of interaction products in depressions, recesses, and corners, fluid movement is forced. After washing off the chemical reagents, rub with a napkin with a small amount of polish.
Electropolishing Stainless Steel: Technical Aspects
Electropolishing is a process by which you can polish a metal surface. It is wrong to think about replacing mechanical cleaning with this process: this technology can be used as a finishing process for small products with irregular and complex shapes. Polishing can provide an excellent support for production because it defines a crystalline structure suitable for welding, the most efficient way for lattice forces to operate. From this point of view, the process is called "glossy etching"
. Like all anodic processes, electrochemical polishing is closely related to the metal-based structure. If there are defects and impurities in it, the electrolytic polishing effect may have spots, dimples and cavities.
The electrolyser shown in Figure 1 explains how the electropolishing process occurs. The stainless steel product used to obtain a mirror surface is determined by the anode, the cathode can be a metal such as lead, copper, etc. During the process, due to the passage of current in certain electrolytic solutions, selective anodic dissolution occurs along the stainless steel surface, which gradually becomes smoother.
Parameters regulating the electropolishing process:
- Current density;
- Voltage;
- Type of electrolytic solution;
- Temperature;
- Stirring liquid;
- Cathode material;
- Size and shape of electrodes;
- Distance between anode and cathode;
- Product location.
All these parameters affect the service life and appearance of the surface of a stainless steel product. For example, the temperature must be kept constant and the mixing must be such that it does not cause local heating.
As can be seen in Figure 2, to achieve proper electropolishing, the electrical parameters must match in the Vc-Vb range. At lower voltages they cause anodic corrosion and parts usually become opaque and corrode. At values higher than Vc, gaseous substances are formed which alter the dissolution process and cause irregular action on the metal surface. The curve in question varies depending on the resistivity of the electrolyte solution. The higher the resistivity, the more the polishing straight part (polishing) will be dense until it is reduced to a point.
Methods and means for polishing stainless steel coatings
The smooth surface of the metal gets damaged when handled carelessly due to deliberate human actions. Not every mark can be removed with stainless steel polish and a soft rag. In a home workshop (garage), many methods are available for processing volumetric, flat, and curved surfaces of alloy alloy products. It is necessary to have the appropriate equipment and reagents.
CLINOX and INVERTER technology
Best results should be maintained at well-defined current density and voltage ratios. This ratio is determined in our CLINOX units, which, thanks to inverter technology, allow control of electrical parameters, increasing electrical efficiency and process reliability. Propaganda is not always accepted. It is often used to prevent uncontrolled heating and local turbulence in a high resistivity electrolytic bath. Mixing should not be too vigorous and can be achieved using passive material or by insufflation of air or nitrogen. The "useful life" of the electrolyte is quite limited. When a certain amount of metal ions appears in the bath, its polishing effect decreases or disappears. So they resort to partial or complete replacement of the used fluid.
Plasma polishing
The technology differs from the electrochemical procedure in the following parameters:
- the solution is not aggressive, disposal does not require special cleaning;
- voltage is higher (220 V);
- temperature is about 100 °C.
The reagent used is ammonium salt with a concentration in the solution of 3.1 ÷ 6.0%. The electric current density is set at 0.35 ± 0.15 A/cm²; gas bubbles are intensively formed in the contact zone of the electrolyte with the stainless steel. Discharges occur in the vapor inside the fluidized bed, ionizing the medium. Plasma tongues appear that specifically act on the steel, polishing it. The time required for one dive is within 6 minutes, based on a power consumption of 5 Wh/cm².
For a stable process of polishing a surface of a certain area using the electroplasma method, the appropriate power of the installation is required. You cannot reduce its value in the hope of increasing the duration of treatment in the bath. The conditions for the formation of a plasma-ionized layer will not be met.
Unscrupulous mechanical preparation will be evident. Residual traces of welds, scratches, and dents cannot be hidden with polish.
Electrochemical polishing of steel
Electrochemical polishing is a procedure for treating the surface of a workpiece by immersing it in an acid solution under the influence of an electric current. It smoothes the surface of the part and allows you to polish metals without the use of paint and varnish coatings. As a result of the interaction of chemical components and electrical charges, reactions are triggered that give the product a mirror shine.
Anode method
Electrochemical treatment reduces the time spent in relation to the mechanical procedure by 4-5 times, increasing the cleanliness class of the mirror by 1 or 2 positions. To polish in this way, the complexity of the joints and the curvature of the planes become unimportant. When electricity is connected, the solution becomes an active electrolyte, interacting more intensely. The sample being processed must be connected to the anode of the installation. For each chemical composition of stainless steel, reagents and mode parameters are selected.
Description of the method
The electrochemical polishing procedure is based on anodic dissolution of the surface of the workpiece. During this process, the protrusions on the surface with a rough topography quickly dissolve. In the depressions of the part, dissolution occurs in a slow manner. The rough side becomes smooth due to the unbalanced dissolution rate, resulting in extra shine.
The process of electrochemical polishing of a part occurs in several stages:
- Manufacturing of electrolytic baths intended for polishing the surface of a product. They contain universal electrolytes: phosphoric acid, sulfuric acid, chromic anhydride and water. When polishing products made from stainless steel, glycerin is additionally used. The creation of baths occurs at temperatures up to 90° C, anode current density up to 80 A/dm 2 and voltage up to 8 V. Electrolytic baths heated to high temperatures pose a danger to human health. If solutions come into contact with the skin, there is a high risk of chemical burns.
- Preparing the workpiece for processing. Products should not have deep patterns or large scratches on their surface that are not subject to electrochemical polishing. It is important that the part is made of soft metals. This parameter influences the degree of effective polishing. The harder the metal, the more difficult it is to achieve a uniform surface when smoothing the rough sides of the workpiece.
- Interaction of the part with electrolyte solutions. In this case, the metal workpiece acts as an anode - an electrode with a positive charge, and the electrolytic bath acts as a cathode. The exposure time of the product in the solution depends on the type of material. Aluminum blanks are kept for 2 - 3 minutes, cast stainless steel parts - up to 30 minutes. As a result of the reaction, roughness gradually smoothes out due to the appearance of a hydroxide or oxide film. Polishing occurs due to the exchange of particles between the anode and the electrolyte. After completion of electrochemical polishing, the surface of the workpiece becomes uniform and acquires a mirror shine.
An easy way to polish stainless steel at home
Polishing stainless steel at home is easy. It all depends on how damaged and oxidized the object being polished is, as well as on the presence of hard-to-reach places. If the stainless steel surface has simply lost its shine due to oxidation, you can use chemical polishing with vinegar, olive oil or special branded products. To do this, you just need to apply one of these substances to a microfiber cloth, then work it on all sides with smooth circular movements. This way you can restore the shine of kitchen equipment, dishes, as well as stainless pipes in the bathroom.
To polish stainless steel products to a mirror shine at home, GOI paste is usually used. Polishing is done using felt or felt. After completion, all surfaces must be cleaned using a microfiber cloth moistened with a small amount of solvent.
Both of these methods are suitable in cases where the stainless steel is not significantly damaged. If there are scratches, gouges and a large amount of plaque before polishing, you will have to mechanically grind the stainless steel (manually or using a power tool).
Popular products
Due to the fact that steel can be easily forged, cut and given the desired shape, the products are in demand in the following industries:
- Medicine - for the manufacture of scalpels, clamps and other medical equipment, furniture;
- Automotive industry;
- Heavy industry;
- Manufacturing of stairs, railings, fences, balconies, terraces;
- Shipbuilding;
- Food Industry;
- Production of stainless steel pipes. Due to polishing outside and inside, the pipes are resistant to the appearance and growth of bacteria. Durable, reliable, resistant to erosion. Suitable for welding and fittings;
- Production of containers for any liquids.
Steel sheets for production
Stainless steel processing process
Processing stainless steel is a difficult process. To make certain objects or parts, it must be cut, bent, and passed through a lathe. During processing of the material, it is necessary to monitor strain hardening, try to quickly remove chips, and take into account the service life of the tools used in the process. Processing stainless steel is a precise process during which certain rules and sequences of actions must be followed. There are other challenges when working with stainless steel.
Viscosity of the material. It is quite plastic, so the chips curl into a spiral of considerable length during cutting.
Low level of thermal conductivity. While this property is an advantage in use, it only gets in the way when processing stainless steel. During cutting, you have to cool the product with special liquids to avoid hardening and heat. Alloy particles remain on the tools, quickly rendering them unusable.
Saving properties. Turning of stainless steel is not accompanied by some loss of strength and hardness due to exposure to high temperatures. Such qualities are inherent in heat-resistant materials. This leads to breakdown of working equipment.
Presence of abrasive compounds. Stainless steel contains microscopic compounds of carbide and intermetals. They impart abrasive properties to the alloy, which damage the cutters.
Uneven hardening . This quality is very difficult when making large parts. They become less quality.
Premises and staff
For work you will need two types of premises:
- an office where samples of finished products are placed;
- manufacturing facility.
It is convenient when the office and production premises are nearby, but not necessary. It is quite possible to choose an office within walking distance of potential buyers, and place production on the outskirts of the city, where rent is lower.
For a workshop with a large volume of output, a room of 200 square meters is suitable. For small structures, 100 squares will be enough. This area houses a production workshop, a warehouse for raw materials, and a staff room. The building must have electricity, heating and water supply. A telephone and internet connection are required. The premises should be located on the ground floor, with convenient access for transport.
At the initial stage of production, 5-10 employees are enough:
- director;
- accountant;
- managers for working with customers and suppliers;
- shop workers;
- technologist;
- loaders, cleaners;
- driver if you plan to export finished products using your own transport.
It is important that the technologist and workshop workers must have appropriate education and work experience, since the production of metal products requires precision and qualifications. As production and sales volumes increase, the number of employees may increase.
Production costs:
| № | Name | cost per year |
| 1 | workshop rental | 350 000 |
| 2 | office rental | 200 000 |
| 3 | purchase of equipment | 3 000 000 |
| 4 | salary costs and taxes | 1 200 000 |
| 5 | consumables, metal | 500 000 |
| Total: | 5 250 000 |