Gaseous
Propane belongs to the organic substances of the alkanes class. Propane is found in natural gas and can be formed during the cracking of petroleum products. Propane is considered one of the most poisonous gases.
Physical properties
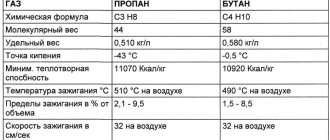
Propane is a colorless gas that is slightly soluble in water. The boiling point of propane is 42.1C. When in contact with air, propane forms an explosive mixture (at a vapor concentration of 2 to 9.5%). At a pressure of 760 mmHg, the combustion temperature of propane can be about 466 °C.
Chemical properties
The chemical properties of propane are similar to most of the properties of a number of alkanes. These properties include: chlorination, dehydrogenation, and so on.
Propane Applications
Propane is widely used as a fuel for various needs. It is an important component of liquefied hydrocarbon gases. Propane is used for the production of solvents and in the food industry (as a propellant, additive E944).
Refrigerant
A mixture of isobutane (R-600a) and pure propane (R-290a) does not harm the ozone layer and has a low greenhouse potential (GWP). Therefore, this mixture is widely used as a refrigerant. This mixture has replaced obsolete refrigerants in refrigeration and air conditioning units.
Butane (C4H10) - like propane, belongs to the class of alkanes. This is an organic compound that is very toxic and causes poisoning to the human body if inhaled. In chemistry, butane is usually called a mixture of n-butane and its isomer isobutane CH(CH3)3. The name butane consists of two parts, the root “but-”, which in English means butyric acid, and the ending “-an”, which indicates that this substance is an alkane.
Isomerism
Butane has two isomers:
melting point, °C
boiling point, °C
Physical properties
Butane is a colorless and flammable gas. At normal pressure and temperatures below 0 °C it liquefies easily. With increased pressure and normal temperature, it is a highly volatile liquid. The solubility of butane in water is 6.1 mg per 100 milliliters of water. Butane at a pressure of 10 atmospheres and a temperature of 100 °C can form an azeotropic compound with water.
Finding and receiving
Butane is found in oil and gas condensate (its share is approximately 12%). Butane is also produced by hydrocatalytic or catalytic cracking of petroleum fractions. In laboratory conditions, butane is obtained by the Wurtz reaction:
Applications and reactions
Free radical chlorination produces a mixture of 2-chlorobutane and 1-chlorine. Combustion in air produces water and carbon dioxide. Butane is widely used as a mixture with propane in lighters and gas cylinders. In them it is in a liquefied state and has a certain odor due to the presence of odorants in the mixture. There are “summer” and “winter” mixtures, which have different compositions. The heat of combustion of one kilogram of butane is approximately 45 MJ (12.72 kWh).
When there is a lack of oxygen, soot or carbon monoxide or both is formed.
DuPont has patented a method for producing maleic anhydride by catalytic oxidation from n-butane
n-Butane is a good raw material for the production of butene, 1,3-butadiene, which are important components of high octane gasoline. Pure butane is used as a refrigerant in refrigeration and air conditioning units. Butane is better than freon due to its environmental friendliness and safety for the environment, but is less productive than freon refrigerants. Butane is registered as food additive E943a in the food industry, and isobutane is registered as additive E943b, propellant. These substances are used in deodorants.
In the food industry, butane is registered as a food additive E943a , and isobutane - E943b , as a propellant, for example in deodorants.
The effect of butane on the human body
Human inhalation of butane can cause heart failure and death from asphyxiation. Contact with liquid butane or a jet of butane gas causes cooling to minus twenty degrees, which is very dangerous for humans.
Methane CH4
also called swamp gas, since it constitutes the main part of flammable gases that rise in bubbles from swamp mud, where methane is formed during the decay of plant residues (cellulose) without access to air. It is also called firedamp because it is formed by the slow decomposition of coal underground and is sometimes released in large quantities in mines; the formation of mixtures of methane with air can cause dangerous explosions. Large quantities of methane are found dissolved in oil; in oil-bearing areas it sometimes comes out of the ground. Methane is also part of lighting gas; Typically, purified illuminating gas obtained by pyrolysis of coal or oil contains about 50% hydrogen, 34% methane, 8% carbon monoxide, 4% unsaturated hydrocarbons, 4% nitrogen and 1% carbon dioxide.
Enormous quantities of methane are contained in some natural gas deposits. Currently, such deposits are being successfully exploited near Saratov, Stavropol, Dashava, etc.; they supply gas consisting of almost pure methane to Moscow, Kyiv and other large cities and industrial centers. In recent years, a number of new deposits of natural methane have been discovered on the territory of the USSR.
Due to the great importance of methane as a substance underlying the most important series of organic compounds, chemists have made great efforts to synthesize methane from elements. Methane was first obtained from carbon disulfide (easily obtained synthetically from elements) by passing it along with hydrogen sulfide through tubes of hot copper (Berthelot, 1856):
Only much later (1897) was it found that methane can be obtained as the only reaction product by the direct combination of carbon with hydrogen at 1200 ° C; in the presence of nickel, this reaction occurs with good yield at a lower temperature (475 °C).
Methane can be produced by the action of water on aluminum carbide:
This is one of the most convenient ways to obtain methane in the laboratory. It is also obtained by the reduction of carbon monoxide or carbon dioxide with hydrogen in the presence of metallic nickel at 250-400 ° C. In addition, methane can be obtained by any of the general methods for producing hydrocarbons, and in laboratories it is often obtained by fusing sodium acetate with caustic soda.
Methane is a colorless, odorless gas, slightly soluble in water, somewhat better in alcohol. In 100 volumes of water at 20°C, approximately 3.3 volumes of methane are dissolved, and at 0°C - approximately 5.5 volumes of methane. This is a constant gas; its critical temperature is -82.1°C at 45.8 at. It burns with a pale bluish flame.
When passing through hot tubes, as well as under the action of a spark electric discharge, methane decomposes into hydrogen and carbon, however, forming a certain amount of more complex hydrocarbons (ethane, ethylene, acetylene, benzene, naphthalene).
By passing a mixture of methane with air through heated tubes with various catalysts, methyl alcohol and formic aldehyde can be obtained as methane oxidation products.
In scattered light, chlorine and bromine replace hydrogen atoms in methane, forming, for example, the compounds CH3Cl, CH2Cl2, CHCl3 and CCl4. Under the influence of direct sunlight, as well as when a mixture of methane and chlorine is ignited, carbon is released and hydrogen chloride is formed according to the equation
Some physical properties of methane are indicated above (see Table 1).
Methane, as the main component of natural gas, is an important industrial raw material for the production of acetylene, chlorine derivatives (from methyl chloride to carbon tetrachloride), formaldehyde and nitro-methane.
Liquefied petroleum gas (LPG), liquefied petroleum gas (LPG)
Liquefied petroleum gas (LPG) is one of the types of alternative fuel.
LPG is a mixture of propane, normal butane, isobutane, propylene, ethane, ethylene and other hydrocarbons.
Methods for obtaining LPG:
- as a product of oil refining at oil refineries (refineries),
- in the production of oil and natural gas.
The use of a mixture of these gases as fuel is due to a number of physical and chemical properties:
- high boiling points at atmospheric pressure - allow you to store a liquefied propane-butane mixture in the operating temperature range: - 40°C - + 45°C at relatively low pressure (up to 1.6 MPa);
- LPG does not lose or change its properties over time and does not erode.
- octane number of LPG is more favorable in comparison with gasoline and diesel fuel and varies in the range of 90 -110, depending on the ratio of propane and butane in the mixture.
- The energy efficiency of LPG is lower than that of traditional fuels due to the low energy per unit. volume. This increases combustion consumption by 10-20% compared to gasoline fuel, but is compensated by 2 times lower price.
- LPG burns more efficiently and safely even in a cold engine, even when the engine is cold, burns relatively cleanly, without smoke and ash, that is, it is more environmentally friendly.
Compared to diesel fuel:
- 90% less particulate matter,
– 90% less nitrogen oxides,
— 60% less carbon dioxide CO2,
— LPG does not pollute the soil because it does not dissolve in water.
Each of the gas components has a certain boiling point, so the pressure of the LPG vapor phase depends on both the temperature and its component composition.
The component composition of liquefied hydrocarbon gas is regulated by GOST 20448-90 “LIQUEFIED HYDROCARBON GASES FOR MUNICIPAL CONSUMPTION”.
The standard provides for 3 brands of gas:
- PT (technical propane),
- SPBT (a mixture of technical propane and butane),
- BT (technical butane).
The content of propane, butane and other impurities in LPG affects many of its properties, because it significantly affects the octane number and vapor density of the fuel.
Octane number (ON) is an indicator of the fuel’s resistance to detonation:
- grows due to an increase in the content of saturated hydrocarbons (propane, n-butane, isobutane, etc.),
- unsaturated hydrocarbons polymerize, which contributes to the formation of sediment - carbon deposits in the tank, in the fuel system and combustion chamber.
Vapor pressure (mixture volatility) is very important in low ambient temperatures. Keeping it at the appropriate level allows the LPG to exit the tank. Both components of the mixture are gaseous and low-boiling.
Propane boils at atmospheric pressure already at -42 ° C, butane, under the same temperature conditions at -0.5 ° C, therefore, in winter, the propane content in the fuel gas is increased to increase the gas vapor pressure.
In summer the mixture ratio is about 40% propane and 60% butane, while in winter the ratio is the opposite: 60/40.
Propane is more expensive than butane, so the “winter” mixture is also more expensive than the “summer” mixture.
Gas stations must monitor the composition of the mixture and not cheat by replacing the winter mixture with a summer one.
Unlike gas filling stations, CNG filling stations use compressed network natural gas from gas pipelines.
LPG production technologies:
- directly from crude oil: during production, associated petroleum gas (APG) is released,
- When stabilized in tanks, ethane, propane, butane and pentane are released.
Ethane C2H6
Ethane C2H6, like methane, is found in oil and in gases released from the ground in oil-bearing areas. It is also found in gases obtained by dry distillation of coal, and in gaseous products of cracking and pyrolysis of oil. In laboratories, ethane is usually obtained by reducing ethyl iodide with zinc dust in an alcohol solution.
or electrolysis of sodium acetate.
Ethane
- a colorless gas that burns with a faintly luminous flame. It can be condensed into liquid already at 4 ° C and a pressure of 46 at. It is almost insoluble in water; 1 volume of absolute alcohol dissolves 1.5 volumes of ethane. At 575-650° C in the absence of catalysts, ethane decomposes into ethylene and hydrogen: