08/14/2020 Author: VT-METALL
Issues discussed in the material:
- Useful and special impurities in steel
- Harmful impurities in steel that impair its properties
Harmful impurities in steel not only worsen its composition, but can also lead to subsequent deformation of a product made from it. However, they cannot all be considered undesirable. Some of them are considered useful, while others cannot be gotten rid of at all, since they are permanent. And there is no need to eliminate them, since permanent impurities can affect the quality characteristics of steel.
In this article we will talk about what harmful impurities are in steel and how they affect its composition and the characteristics of steel products.
Useful and special impurities in steel
Steel contains harmful and beneficial impurities. First, let's look at the useful ones, which include manganese and silicon:
- Manganese is a chemical element that increases the hardenability of steel and reduces the influence of sulfur, which has a harmful effect on the metal.
- Silicon - an admixture of this element helps to deoxidize steel and, as a result, increase its strength. It is specially added to the metal during its smelting.
Carbon steel contains an admixture of silicon of no more than 0.35–0.4% and manganese in an amount of 0.5–0.8%. The transition of manganese and silicon into steel occurs during deoxidation during smelting. These chemical elements combine with the oxygen of iron oxide FeO, and then, turning into oxides, pass into slag, that is, in other words, they deoxidize the steel.
This process has a beneficial effect on the properties of steel. Due to degassing of the metal by silicon, its density increases. Part of the chemical element remains in the ferrite (solid solution) after deoxidation, which leads to a significant increase in the yield strength. At the same time, the ability of steel to cold heading and drawing is reduced.
Recommended reading
- Cutting copper with a laser: advantages and disadvantages of technology
- Types of metal cutting: industrial applications
- Metalworking according to drawings: convenient and profitable
For this reason, manufacturers are reducing the amount of silicon in steels produced for cold forming and heading. The strength of the metal is significantly increased due to the admixture of manganese. The latter greatly reduces the red brittleness of steel, leaving ductility almost unchanged. Thus, the brittleness of steel sharply decreases when exposed to high temperatures, which arose due to the presence of sulfur.
To obtain steels with certain properties, special impurities are added to the metal. They are called alloying elements. Steels are called alloyed steels.
Let us dwell in detail on the purpose of some elements:
- Aluminum - its admixture helps to increase the scale and heat resistance of steel.
- Copper – increases the resistance of steel to corrosion.
- Chromium – increases the strength and hardness of steels, increases resistance to corrosion, while ductility decreases slightly. What makes stainless steel stainless is its high chromium content.
- Nickel – increases ductility, strength, and makes steel resistant to corrosion.
- Tungsten – when added to steel, creates corbides (chemical compounds of increased hardness). They significantly increase red resistance and hardness. Under the influence of tungsten, steel stops expanding during heating, and its brittleness disappears when tempered.
- Vanadium – contributes to an increase in the density, strength and hardness of steel. It is recognized as an excellent deoxidizing agent.
- Cobalt - under its influence, heat resistance, resistance to shock loads increases, and magnetic properties increase.
- Molybdenum – steel’s resistance to oxidation improves when exposed to high temperatures, elasticity and red-hardness increases, corrosion resistance increases, and tensile strength increases.
- Titanium - being an excellent deoxidizer, it increases resistance to corrosion, increases the density and strength of the metal, and improves its workability.
- Cerium – helps to increase the ductility and strength of steel.
- Zirconium (Z) - affects the grain size of steel, making it possible to produce metal with a specified grain size, making it finer.
- Lanthanum, neodymium and cesium - reduce the porosity of steel, reduce the amount of sulfur, make the surface quality better and the grain finer.
Impurities: permanent, hidden and random
Manganese, silicon, aluminum, sulfur and phosphorus
are classified as
permanent impurities
.
Aluminum, together with manganese and silicon, is used as a deoxidizer and therefore they are always present in small quantities in deoxidized steels. Iron ores, as well as fuels and fluxes, always contain a certain amount of phosphorus and sulfur, which remain in cast iron and then pass into steel
.
Nitrogen
called
a hidden
impurity - it enters steel mainly from the air.
To random
impurities include
copper, arsenic, tin, zinc, antimony, lead
and other elements. They end up in the steel with the charge - with ores from various deposits, as well as from iron scrap.
All impurities - permanent, hidden and accidental - are inevitable to varying degrees due to steel production technology. Thus, mild steel usually contains these impurities within the following limits: 0.3-0.7% manganese; 0.2-0.4% silicon; 0.01-0.02% aluminum; 0.01-0.05% phosphorus, 0.01-0.04% sulfur, 0.-0.2% copper. In these quantities, these elements are considered as impurities, and in larger quantities, which are intentionally added to steel, they are already considered alloying elements.
Harmful impurities in steel that impair its properties
Let's figure out what harmful impurities are contained in steel. The main ones are phosphorus and sulfur.
- Sulfur.
Sulfur (S) is contained in high quality steels in an amount of no more than 0.02–0.03%. For general purpose metal, this figure rises to 0.03–0.04%. Using special treatment, the amount of sulfur is reduced to 0.005%.
Sulfur does not dissolve in iron, but FeS (iron sulfide) is formed. It enters the eutectic formed at a temperature of +988 °C.
With a high sulfur content, steel becomes red brittle. This is due to the appearance of sulfide eutectics at the grain boundaries, which have low melting ability. Red brittleness appears at the red hot temperature of steel – +800 °C.
Sulfur has a bad effect on weldability, ductility, impact strength, and the surface of the metal. This is especially noticeable if manganese and carbon are present only in small quantities.
VT-metall offers services:
Sulfur has a significant tendency to segregate at grain boundaries. For this reason, during heating, the ductility of steel decreases. If the metal is intended for further processing by an automatic mechanical method, then sulfur must be added to the composition in an amount of 0.08% to 0.33%, since it helps to increase the fatigue strength of bearing steels.
Manganese reduces the harmful effects of sulfur on steel. When the alloy is in a liquid state, it reacts to form manganese sulfide, the melting point of which is +1620 °C. It significantly exceeds the temperature of hot metal processing (from +800 °C to +1200 °C). With such heating, manganese sulfides are quite plastic and simply deform.
- Phosphorus.
Segregation of phosphorus (P) to a much lesser extent than sulfur and carbon occurs during the solidification of steels. It dissolves in ferrite, which increases the strength of the metal. The higher the percentage of phosphorus the steel contains, the higher its cold brittleness and the lower its impact strength and ductility.
The high temperature of the environment makes it possible to achieve phosphorus solubility within 1.2%. The lower the temperature becomes, the lower the solubility of phosphorus. It gradually drops to 0.02–0.03%. It is this content of this chemical element that is observed in steels. This may indicate that it tends to be completely soluble in alpha iron.
The temper brittleness of chromium, chromium-nickel and chromium-manganese, manganese and magnesium-silicon alloy steels largely depends on the segregation of phosphorus along the grain boundaries. The element helps slow down the decomposition of martensite and increases hardening.
The influence of aluminum on the properties of steels
Aluminum (Al) is widely used for deoxidation of liquid steel, as well as for grain refinement of steel ingots. The harmful effects of aluminum include the fact that it promotes the graphitization of steels. Although aluminum is often considered an impurity, it is also actively used as an alloying element. Because aluminum forms solid nitrides with nitrogen, it is usually an alloying element in nitrided steels. Aluminum increases the resistance of steels to scaling, and therefore it is added to heat-resistant steels and alloys. In dispersion-hardening stainless steels, aluminum is used as an alloying element that accelerates the dispersion precipitation reaction. Aluminum increases the corrosion resistance of low-carbon steels. Of all the alloying elements, aluminum is the most effective for controlling grain growth when heating steels for hardening.
The influence of nitrogen on the properties of steels
The harmful effect of nitrogen (N) is that the rather large, brittle non-metallic inclusions it forms - nitrides - worsen the properties of steel. A positive property of nitrogen is that it is capable of expanding the austenitic region of the phase diagram of steels. Nitrogen stabilizes the austenitic structure and partially replaces nickel in austenitic steels. Nitride-forming elements vanadium, niobium and titanium are added to low-alloy steels. When controlled by hot working and cooling, they form fine nitrides and carbonitrides, which significantly increase the strength of the steel.
The influence of copper on the properties of steels
Copper (Cu) has a moderate tendency to segregate. The harmful effects of copper include a decrease in the cold brittleness of steel. With an increased copper content, it negatively affects the quality of the steel surface during hot processing. However, with a content of more than 0.20% copper increases its resistance to atmospheric corrosion, as well as the strength properties of alloy and low-alloy steels. Copper in amounts greater than 1% increases the resistance of austenitic stainless steels to sulfuric and hydrochloric acids, as well as their resistance to stress corrosion.
Phosphorus
Iron ores, fuels, and fluxes contain some amount of phosphorus, which during the production of cast iron remains in it to one degree or another and then turns into steel. Phosphorus dissolves well in ferrite and austenite, and at high content it forms phosphide Fe3P (15.62% P). Dissolving in ferrite, phosphorus distorts the crystal lattice and increases the strength and yield limits of steel, greatly reducing ductility and toughness; every 0.01% P increases the cold brittleness threshold by 20...25 0 C. Phosphorus is a harmful impurity in steels.
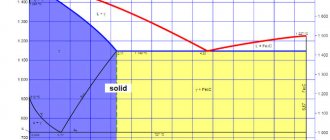
Like phosphorus, sulfur enters the metal from ores, as well as from furnace gases - a product of fuel combustion (SO2). Sulfur is very limitedly soluble in ferrite, and almost any amount of it forms a sulfur compound with iron - iron sulfide FeS, which is part of the eutectic, which has a melting point of 988 0 C. It is located mainly along the grain boundaries. When steel is heated to rolling or forging temperatures (1000...1200 0 C), the eutectic melts, breaking the bond between grains. During deformation, tears and cracks form in these places. This phenomenon is called red brittleness. The introduction of manganese into steel reduces the harmful effects of sulfur, since when it is introduced into liquid steel, manganese sulfide is formed, which has a melting point
FeS + Mn -> MnS + Fe.
MnS particles are located in the form of separate inclusions and, during deformation, are pulled into lines along the rolling.
Sulfur compounds greatly reduce the mechanical properties of steel under static and cyclic loading, especially toughness, ductility, and endurance limit. Sulfur is a harmful impurity in steels.
The influence of chemical composition on the mechanical properties of steel
Each chemical element that makes up steel affects its mechanical properties in its own way - improves or worsens.
Carbon (C), which is an essential element and is usually found in steel in the form of the chemical compound Fe3C (iron carbide), increases its content to 1.2%, increases the hardness, strength and elasticity of steel and reduces toughness and weldability. This also reduces machinability and weldability.
Silicon (Si) is considered a beneficial impurity and is introduced as an active deoxidizer. As a rule, it is contained in steel in small quantities (up to 0.4%) and does not have a noticeable effect on its properties. But when the silicon content is more than 2%, the steel becomes brittle and breaks during forging.
Manganese (Mn) is contained in ordinary carbon steel in small quantities (0.3-0.8%) and does not have a serious effect on its properties. Manganese reduces the harmful effects of oxygen and sulfur, increases the hardness and strength of steel, its cutting properties, increases hardenability, but reduces resistance to impact loads.
Sulfur (S) and phosphorus (P) are harmful impurities. Their content, even in small quantities, has a harmful effect on the mechanical properties of steel. A steel content of more than 0.045% sulfur makes the steel red-brittle, i.e. one that cracks when forged in a heated state. Steel is protected from red brittleness by manganese, which binds sulfur into sulfides (MnS). The content of phosphorus in steel is more than 0.045%, making the steel cold-brittle, i.e. easily broken when cold. Phosphorus somewhat improves the machinability of steel, as it promotes chip separation.
Niobium (Nb) improves the acid resistance of steel and helps reduce corrosion in welded structures.
Titanium (Ti) increases the strength, density and ductility of steel, improves machinability and corrosion resistance. Increases the hardenability of steel at low contents and decreases at high contents.
Chromium (Cr) increases strength, hardenability and heat resistance, cutting properties and abrasion resistance, but reduces the toughness and thermal conductivity of steel. The content of a large amount of chromium (in ordinary types of steel reaches 2%, and in special types - up to 25%) makes the steel stainless and ensures the stability of magnetic forces.
Molybdenum (Mo) increases the strength characteristics of steel, increases hardness, red resistance, and anti-corrosion properties. Makes it heat-resistant, increases the load-bearing capacity of structures under shock loads and high temperatures. It makes welding difficult because it actively oxidizes and burns out.
Nickel (Ni) increases toughness, strength and elasticity, but slightly reduces the thermal conductivity of steel. Nickel steels are easy to forge. The significant nickel content makes the steel non-magnetic, corrosion-resistant and heat-resistant.
Tungsten (W), forming solid chemical compounds in steel - carbides, sharply increases hardness and red-hardness. Increases the performance of steel at high temperatures, its hardenability, increases the resistance of steel to corrosion and abrasion, and reduces weldability.
Vanadium (V) provides fine-grained steel, increases hardness and strength. Increases the density of steel, as it is a good deoxidizer. Reduces the sensitivity of steel to overheating and improves weldability.
Cobalt (Co) increases heat resistance, magnetic properties, and increases impact resistance.
Aluminum (Al) is an active deoxidizer. Makes steel fine-grained, homogeneous in chemical composition, prevents aging, improves stampability, increases hardness and strength, and increases oxidation resistance at high temperatures.
Copper (Cu) increases corrosion resistance, yield strength and hardenability. Does not affect weldability.
For a comprehensive understanding and analysis of the processes occurring during alloying and deformation of steels, knowledge of the relationships between the chemical composition and mechanical properties plays an important role.
The purpose of this research is to study the complex effect of chemical composition on the yield strength σT of reinforcing steel of class A500C
.
During September and October of this year, samples of reinforcing bars with a diameter from Ø16 to Ø36 were tested in the Laboratory for Testing Building Materials and Structures of the State Budgetary Institution “TsEIIS”. More than 30 parallel tests were performed. At the same time, for the same sample of a given standard size of reinforcing bars, the actual mass fraction of chemical elements was determined using a PMI-MASTER SORT optical emission spectrometer (Fig. 1) and the mechanical properties of steel using an IR-1000M-auto testing machine (Fig. 2 ).
Fig. 1 - Testing a reinforcing bar to determine the chemical composition of steel.
Fig. 2 - Tensile testing of reinforcing steel.
To ensure the reliability of statistical conclusions and meaningful interpretation of research results, the required sample size was first determined, i.e. minimum number of parallel tests. Since in this case the tests are carried out to assess the mathematical expectation, then with a normal distribution of the value under study, the minimum required volume of tests can be found from the relationship:
where υ is the sample coefficient of variation,
tα,k – Student coefficient,
α=1-P – significance level (P – confidence probability),
k = n-1 – number of degrees of freedom,
ΔМ – maximum relative error (tolerance) when estimating the mathematical expectation in fractions of the mathematical expectation (ΔМ = γ*δМ, where γ is the general coefficient of variation, δМ is the maximum error when estimating the mathematical expectation in fractions of the standard deviation).
As a rule, the general coefficient of variation γ is unknown, and it is replaced by the sample coefficient of variation υ, to determine which we conducted a series of ten preliminary tests.
Based on the results of the tests and calculations performed with a confidence probability of P = 0.95, the required sample size was obtained equal to n = 26
. The actual number of tests, as mentioned above, was 36.
The array of data obtained from the results of parallel tests was processed using multivariate correlation analysis.
The multiple regression equation can be represented as:
Y = f (β, X) + ε,
where X=(X1, X2,…, Xm) – vector of independent (initial) variables; β – vector of parameters (to be determined); ε – random error (deviation); Y – dependent (calculated) variable.
The development of a multiple correlation model is always associated with the selection of significant factors that have the greatest impact on the outcome trait. In our case, three elements (Al, Ti, W) were excluded from further consideration due to their low mass fraction (
If you find an error: select the text and press Ctrl+Enter