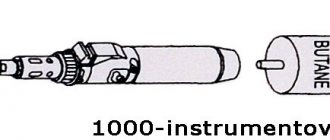
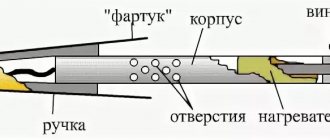
Anyone who has at least once tried to unsolder a microcircuit with a soldering iron has probably experienced certain difficulties. This is explained by the fact that in order to desolder a large number of legs, it is necessary to either warm them all up at the same time, or free them from solder one by one.
Only in this case is it possible to keep the contacts on the board in good condition, which allows you to subsequently solder in a new chip. If you are not completely sure that an expensive part is faulty, it is natural to want to keep it in working order without overheating during dismantling.
Features of dismantling
There are many known technical techniques that allow you to solder a microcircuit with a soldering iron, each of which has its own advantages and disadvantages.
You can remove electronic parts from boards without damaging the contacts in the following ways:
- by heating the soldering areas with just a soldering iron (with the addition of flux);
- through a special suction that removes molten solder from the contact pads;
- using a metal braid from a coaxial cable applied to the soldered leg;
- using heat-conducting metal plates (blades) or copper attachments that have slots for the contact patches of microcircuits.
The first three methods are suitable if you have a soldering iron whose power exceeds 25 watts.
The option of using special attachments involves replacing the working tip and is only suitable in combination with “powerful” soldering stations (more than 40 watts), capable of heating it together with the contacts soldered into the board.
In addition, this method of desoldering a part is only suitable for microcircuits with a suitable arrangement of legs for the configuration of the nozzle. The approach that has become more widespread is when a regular razor blade is used as a heater.
How to distinguish zinc from other metals - Metalist's Handbook
Zinc or Zincum is element 30 of Mendeleev's periodic table of chemical elements and is designated by the symbol Zn.
It is mainly used in the creation of deformed semi-finished products and as part of various types of mixtures.
In its pure form, it looks like a brittle metal of a bluish-silver color; it quickly oxidizes and becomes covered with a protective film (oxide), due to which it noticeably tarnishes.
It is mined in Kazakhstan, Australia, Iran and Bolivia. Due to the difficulties in identifying the metal, it is often called "blende" .
Historical reference
The name “zinc” itself was first mentioned in the book “Liber Mineralium” by Paracelsus. According to some sources, it meant “prong.” The alloy of zinc with copper or brass has been known for a long time. It was used in Ancient Greece, India and Ancient Egypt, and later the material became known in China.
The metal was obtained in its pure form only in the first half of the 18th century in 1738 in Great Britain using the distillation method. Its discoverer was William Champion.
Industrial production began 5 years later, and in 1746 in Germany, the chemist Andreas Sigismund Marggraff developed and described in detail his own method for producing zinc . He proposed using the method of calcining a mixture of metal oxide and coal in fireproof clay retorts without air access.
Subsequent condensation of the vapors had to take place in the refrigerator. Due to his detailed description and painstaking development, Marggraf is often called the discoverer of the substance.
At the beginning of the 19th century, a method was found for isolating metal by rolling at 100 C o -150 C o. At the beginning of the next century, they learned to extract zinc using the electrolytic method. In Russia, the first metal was produced only in 1905.
Physical properties
- Atomic number: 30.
- Atomic mass: 65.37.
- Atomic volume: 9.15
- Density: 7.133 g/cm3.
- Temperature required for melting: 419.5 C o.
- Boiling point: 906 C o.
- Surface energy: 105 mJ/m2.
- Specific electrical conductivity: 16.2*10-6 S/m.
- Molar heat capacity: 25.4 J/(K*mol).
- Molar volume: 9.2 cm3/mol.
Zinc has weak mechanical properties; at normal temperatures it easily breaks and crumbles, but at temperatures of 100 C o -150 C o it becomes quite malleable and is easily deformed: it is forged and rolled into sheets. Plain water is safe for metal, but acids and alkalis easily corrode.
Because of this, zinc in its pure form is not used for the manufacture of parts, only alloys.
Chemical properties
The external electronic configuration of one zinc atom can be written as 3 d 104 s 2. The metal is active and is an energetic reducing agent. At a temperature of 100 C, it becomes covered in open air with a film consisting of basic carbonates and becomes very dull.
When exposed to carbon dioxide and high humidity, the element begins to deteriorate. In an oxygen or normal environment, when exposed to high heat, zinc burns, producing a bluish flame and white smoke, which consists of zinc oxide.
The dry elements of fluorine, bromine and chlorine have a flammable effect on zinc, but only with the participation of water vapor.
When a metal and strong mineral acids combine, the former dissolves, especially if the mixture is heated, resulting in the formation of the corresponding salts .
Alkalis, melts and solutions oxidize the substance, resulting in the formation of zincites, soluble in water, and hydrogen is released. The intensity of the effects of acids and alkalis depends on the presence of impurities in zinc.
The more “clean” the metal, the weaker it interacts due to hydrogen overvoltage.
in nature
Zinc does not occur as an independent element in nature. It can be extracted from 66 minerals, including sphalerite, calamine, franklinite, zincite, willemite, and smithsonite.
The former is the most common source of the metal and is often called "zinc blende". It consists of zinc sulfide and impurities that give the mineral its varied colors.
This makes it difficult to find and correctly identify.
You can find zinc in acidic and igneous rocks - in the latter there is a little more of it. Often the metal in the form of sulfide, together with lead is found in thermal waters and migrates in surface and underground sources.
Features of smelting
The temperature required to melt zinc should be less than 419 C o, but not more than 480 C o. Otherwise, metal waste will increase and wear on the walls of the bath, which is usually made from iron, will increase.
In the molten state, no more than 0.05% iron admixture is allowed, otherwise the temperature required for melting will begin to rise. If the percentage of iron content exceeds 0.2%, zinc cannot be rolled.
Zinc is obtained from polymetallic ores, which can contain up to 4% of the element .
If the ores have been enriched by selective flotation, up to 60% of zinc concentrates can be obtained from them, the rest will be occupied by concentrates of other metals.
Zinc concentrates are fired in furnaces in a fluidized bed, after which zinc sulfide turns into oxide and sulfur dioxide is released. The latter is consumed: sulfuric acid is obtained from it.
To convert zinc oxide into the metal itself, two methods are used.
- Distillation or pyrometallurgical. The concentrate is fired, then sintered to impart gas permeability and granularity and reduced with coke or coal at temperatures of 1200-1300 C°. During the reaction, metal vapors are formed, which are condensed and poured into molds. The purity of zinc reaches 98.7%, after which it can be increased to 99.995% using rectification, but the latter method is quite expensive and complex.
- Electrolytic or hydrometallurgical. The fired concentrates are treated with sulfuric acid, the solution is cleaned of impurities using zinc dust and subjected to electrolysis in bathtubs lined with lead or vinyl plastic. Zinc is deposited on aluminum cathodes, from where it is collected and melted in induction furnaces. The purity of the metal obtained by this method reaches 99.95%.
Mixtures and alloys
To enhance strength and increase the melting point, the metal is mixed with copper, aluminum, tin, magnesium and lead.
The most famous and sought after alloy is brass. This is a mixture of copper with the addition of zinc, sometimes tin, nickel, manganese, iron, and lead are also found. The density of brass reaches 8700 kg/m3 .
The temperature required for melting is kept at around 880 C o - 950 C o: the higher the zinc content in it, the lower it is.
The alloy perfectly resists unfavorable external environments, although it turns black in air if not varnished, it is perfectly polished and welded by resistance welding.
There are two types of brass:
- Alpha brass: more ductile, bends well in any condition, but wears out more.
- Alpha+beta brass: deforms only when heated, but is more wear-resistant. Often alloyed with magnesium, aluminum, lead and iron. This increases strength, but reduces ductility.
Zamak or Zamac alloy is composed of zinc, aluminum, copper and magnesium . The name itself is formed from the first letters of the Latin names: Zink - Aluminum - Magnesium - Kupfer / Cuprum (Zinc-Aluminum-Magnesium-Copper). In the USSR, the alloy was known as TsAM: Zinc-Aluminum-Copper.
Actively used in injection molding, melting begins at low temperatures (381 C o - 387 C o) and has a low coefficient of friction (0.07).
It has increased strength, which makes it possible to produce products of complex shapes that are not afraid of breaking: door handles, golf clubs, firearms, construction fittings, fasteners of various types and fishing tackle.
If the temperature required to melt a “pure” metal is 419.5 C o , then the alloy with tin is reduced to 199 C o, and with tin and lead - to 150 C o.
And although such alloys can be soldered and welded, most often mixtures with zinc are used only to seal existing defects due to their weak strength. For example, an alloy of tin, lead and zinc is recommended for use only on nickel-plated products.
Most often, zinc alloys are used to create carburetors, speedometer frames, radiator grilles, hydraulic brakes, pumps and decorative elements, parts for washing machines, mixers and kitchen equipment, watch cases, typewriters, cash registers and household appliances. These parts cannot be used in industrial production: when the temperature rises to 100 C, the strength of the product decreases by a third, and hardness by almost 40%. When the temperature drops to 0 C, zinc becomes too brittle, which can lead to breakage.
Application
Zinc is one of the most popular metals in the world: it is in third place in terms of production among non-ferrous metals, second only to copper and aluminum. This is also facilitated by its low price. It is most often used for corrosion protection and as part of an alloy such as brass.
- In metallurgy, zinc is especially valuable. It is applied in a thin layer to the steel surface of many metal structures to completely protect them from rust on a mechanical and chemical level. Up to 40% of all production is spent on this. Since zinc, unlike nickel, cobalt, tin and cadmium, is more active than iron, it is the first to come into contact with an unfriendly external environment, completely protecting the base.
- Pure metal is used to recover precious metals after mining by leaching. It is also used to extract gold and silver from rough lead.
- Zinc is the most electropositive metal and practically does not react to water. This made it possible to create a large number of different chemical current sources: zinc-air, silver-zinc, mercury-zinc, “dry” Leclanchet elements.
- Zinc dust is used in fireworks and pyrotechnics to create blue fire, and in paint, especially zinc white, for anti-corrosion protection and better adhesion to the substrate. It is also used to displace precious metals from cyanide solutions and to purify zinc sulfate solutions from cadmium and copper.
- In printing, zinc is used for casting fonts and printing illustrations: zincography has been used since the 19th century. In this case, the printing plate is prepared on a zinc base with a small - no more than 5% - addition of other metals. Before each etching, the plate is annealed and rolled in a heated state.
- In medicine, zinc oxide is used as an antiseptic in the ointments “Lassara Paste”, “Sudocrem”, “Zinc Ointment”, as well as as a powder, toothpastes and material for cementing teeth. Metal is used to create bactericidal ceilings and self-cleaning surfaces. Previously, zinc was used for photocatalytic water purification on an industrial scale.
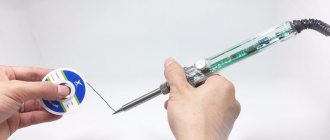
One soldering iron
You can unsolder parts from the board with a regular soldering iron if you grab the solder with a tip dipped in flux.
The essence of this well-known method is that after removing the next portion of molten tin, it is shaken off or wiped on a damp cloth. With each approach, the tip tip is re-wetted with a brush with fresh flux, after which the next portion of the melt is captured. Before wetting, it is recommended to warm the tip well in regular soldering rosin.
To smoothly remove parts with a large number of contacts (excluding planar microcircuits), this operation must be repeated several times. When performing this, you need to ensure that the contact patches do not overheat and subsequently come off along with the legs.
After the bulk of the solder has been removed from the connecting pads, it will be possible, with a little effort, to pry the microcircuit from the side of the board and separate it, desoldering it completely.
Using a razor blade
The main problem with soldering microcircuits is that they have several legs, which is why when one of them heats up, the others have time to cool down. This inconvenience can be overcome by using a heat-conducting device that contacts several legs at once.
In this case, the thermal power of the tip is distributed evenly between them and ensures that the solder melts in several contact areas at once. A simple razor blade can be used as such a device; to heat it up, you will need a soldering iron of suitable power or a hot air gun.
When heating the steel blade, it is recommended to slightly rock the chip on the soldered side, after which you can forcefully pull it out of the board. In the same way, the second row of legs is freed from solder.
Using a special braid
Removing microcircuits with a soldering iron is based on the ability of its tip to attract solder. This is explained by the fact that a high-quality tinned and flux-treated tip is characterized by increased wettability (that is, it grips solder well when soldering).
This effect can be enhanced by using the braid removed from the coaxial cable. Its role can be played by the screen from the antenna wire, removed from it and generously moistened with flux.
If you press the unbraided “braid” of the screen to the contact patch, and then “walk” over this place with a soldering iron, you can observe an interesting effect. Due to the porosity and high hygroscopicity of the braided structure, it absorbs solder well, gradually freeing the chip body with legs.
Dismantling by suction
This method of soldering microcircuits and other small parts is based on the principle of liquid suction by creating a vacuum in the contact area.
Vacuum, in turn, can be created using the following tools:
- a special device that operates on the principle of a bicycle pump (it is called a destin pump);
- suction in the form of an enema, which can be combined with a soldering iron and used simultaneously with heating the contact pad.
Suction structures can have a variety of designs (in the form of a piston with a rod, for example), but their essence does not change. They have been and remain the most effective means of removing liquid solder.
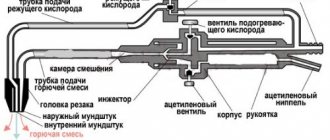
Plasma Cutting Basics
Plasma cutting is based on ionized gas, which flies out of the torch nozzle at high speed. This gas is that same plasma. What is she doing.
- Essentially, this ionized medium is an excellent conductor of electric current, which flows from the electrode to the metal workpiece.
- The plasma heats the metal to the required temperature.
- It blows away the molten metal and frees up the cutting space.
This means that to create plasma, you need gas and a source of electricity. And these two components must come together in one place. Therefore, plasma cutting equipment consists of a gas cylinder, a high-power source of electricity and a cutter in which the electrode is installed.
The design of the cutter is made in such a way that gas passes around the electrode and, when heated from the electrode, escapes out through a small hole. The small diameter of the hole and gas pressure create the necessary speed for the plasma. When making homemade plasma cutting, you just need to purchase a ready-made cutter and not think about creating it. Because everything is already thought out in it, plus the factory version is a guarantee of safety.
As for gas, all options have long been abandoned, leaving compressed air. You can get it today very simply - purchase and install a compressor.
There are certain conditions that guarantee the quality of plasma cutting.
- The current strength at the electrode should not be less than 250 A.
- Compressed air must be supplied to the cutter at a speed within 800 m/sec.
Use of medical needles
In the absence of a special suction device, a novice master can use a medical needle to remove the microcircuit. It must be thin enough to fit into the hole being vacated. At the same time, the needle must have a thickness that allows it to be put on the soldered leg.
Before starting operations, you need to file the tip so that the oblique cut turns out straight, and then flare it a little.
Soldering the part with the resulting device is not difficult at all. To do this, you first need to put the needle all the way onto the pin of the microcircuit, and then use a soldering iron to heat it together with the contact.
While the solder is in the liquid phase, slightly turning the needle, you should push it into the mounting hole (it is advisable to continue rotating until the melt sets).
Upon completion of this procedure, the end of the needle along with the leg will be isolated from the board. Do the same with the remaining legs, after which the microcircuit is unsoldered and removed without any difficulty.
Useful tips
There are many points that necessarily affect the operation of the unit.
- There is no need to purchase, for example, a large compressor. But 2-2.5 atmospheres may not be enough for a large amount of work. The way out is to install a receiver on the compressor. It works like an accumulator that accumulates pressure in compressed air. For this purpose, you can use, for example, bolts from the brake systems of heavy vehicles. The option is actually simple. The volume of the cylinder is large, and it should be enough for a long period of time.
- In order for the air pressure to be stable and uniform, a reducer must be installed at the receiver outlet.
- Of course, the optimal solution is to purchase a compressor complete with a receiver. It costs more than usual, but if this unit is used for other things, for example, for painting, then you can increase its functionality and thereby cover the costs.
- To make a mobile version of the machine, you can make a small trolley. After all, all the elements of a plasma cutter are small devices. Of course, you will have to forget about mobility if the machine is made on the basis of a welding transformer. It's too big and heavy.
- If you can’t buy a ready-made hose-cable kit, you can make it yourself. You need to combine the welding cable and high-pressure hose into one sleeve and place them in a single sheath. For example, into a regular hose of larger diameter. A set made in this way simply will not get in the way, which is very important when cutting metals.
Making your own plasma cutter is not difficult at all. Of course, you will need to obtain the necessary information and study it; it is definitely recommended to watch the training video. And after that, correctly select all the elements exactly to the required parameters. By the way, the assembled plasma cutter based on a serial inverter makes it possible not only to carry out plasma cutting of metals, but also plasma welding, which increases the functionality of the unit.
Use of Rose alloy
You can also unsolder and remove the microcircuit from the board using special compounds called “Rose” or “Wood” alloys. Their distinctive feature is their low melting point (no more than 100 degrees).
Before soldering the microcircuits using this method, several granules of the selected composition are poured directly onto their contacts. After this, using a well-heated soldering iron, a bath of solder is made, spreading evenly over all the legs.
Thanks to the action of the granules, the overall melting temperature in the melt bath will also decrease, which will lead to uniform spreading of liquid solder over the entire plane of the contact pads. In such a heated state, you need to try to pull the microcircuit out of the socket by grabbing it with tweezers.
Analyzing the methods of dismantling microcircuits, it can be noted that all of them can be implemented at home (watch the video). To do this, you only need appropriate preparation, which consists of making the necessary tools with your own hands and purchasing the necessary compounds.
Safety regulations
In fact, unscrewing a broken thread is quite a dangerous activity. Since the hole can be not only through, but also difficult to access.
Let's study the basic rules that need to be followed:
- Protect the skin of your hands and face - substances such as solvents, for example, contain many chemical elements. When mixed, they release substances that, upon close contact, can settle on the mucous membrane. In case of contact with human skin, there is a risk of burns. Be careful if you use this method, as the drops may get into your face or eyes.
- Use a respirator - the smell emanating from caustic substances can also enter the respiratory tract, which can also cause irrevocable harm to a person. But a respirator can trap the smell.
- Wear glasses - if dust and grains of chemical elements get on the mucous membrane of your eyes, they can cause burns or damage your eyes. Moreover, at the preparatory stage this will help you a lot.
If dust or paint does get in somewhere, follow these instructions:
- Rinse the area where the pollen has landed. If you find deep damage, immediately seek medical help from specialists.
- If you swallow dust, you should immediately leave the room for fresh air and try to cough. Be sure to rinse your nose. If you start to feel unwell or experience pain in your lungs, immediately call an ambulance and seek help from specialists.
Before you begin such manipulations, carefully consider your workplace and escape routes. This is necessary in case something happens. You will immediately know what exactly to do and what to get from where.