Brazing
TO
category:
Soldering
Brazing
Next: Adhesive joints
Brazing is used to produce strong and heat-resistant seams.
Soldering with hard solders is carried out observing the following basic rules: - as with soldering with soft solders, the surfaces are adjusted to each other by sawing, thoroughly cleaned of dirt, oxides and fats by mechanical or chemical means; - the fitted parts at the junction are coated with flux, pieces of solder (copper plates) are placed on the junction and secured with soft knitting wire; — prepared parts (blanks) are heated with a blowtorch, in a forge or electric furnace; - when the solder melts, remove the part from the heat and hold it in such a position that the solder cannot flow from the seam; — then the part is slowly cooled. It is impossible to cool parts with a soldered plate in water, as this will weaken the strength of the connection.
Another soldering method is used: the prepared part (product) is heated and sprinkled with borax, then heated and the end of a copper or brass wire is brought to the joint, which, melting, fills the junction. As they cool, the soldered parts are washed in water, wiped with dry rags and dried; The seam is cleaned with sandpaper or filed with a file.
Soldering defects, their causes and preventive measures are as follows:
solder does not wet the surface of the metal being soldered due to insufficient flux activity, the presence of an oxide film, grease and other contaminants. To prevent non-wetting, fluoride salts are added to the flux or its quantity is increased, improving the processing of parts by removing traces of corrosion and grease; solder sagging or dripping due to insufficient heating of the part; the solder has not melted.
Rice. 1. Tinning of parts: a - immersion in a bath of tin, b - heating of parts for tinning, c - maintenance by rubbing tin
Occupational safety when performing soldering and tinning. Workplaces intended for soldering small parts must be equipped with local exhaust devices that provide an air speed directly at the soldering site of at least 0.6 m/s.
In rooms where soldering work was carried out, the floors must be washed; dry cleaning of the floor is not permitted. Storing clothing in rooms where soldering is carried out is prohibited.
In the immediate vicinity of workplaces intended for soldering small parts with soft solders, the following should be installed: a washbasin, a tank with a 1% solution of acetic acid for preliminary washing of hands and easy-to-clean portable containers for collecting paper or cotton napkins and rags . There should always be soap, brushes, and napkins for wiping hands near the sink. Shared towels are not permitted.
The preparation of metals and the soldering process are associated with the release of dust, harmful fumes of non-ferrous metals and salts, which, when entering the human body through the respiratory organs, esophagus or skin, cause irritation of the mucous membrane of the eyes, skin damage and poisoning.
Therefore, when soldering and tinning, the following rules must be observed; The soldering workplace must be equipped with local ventilation; work in gas-polluted areas is not allowed; after finishing work and before eating, wash your hands thoroughly with soap; Add chemicals carefully in small portions, avoiding splashes.
Acid getting into the eyes can cause blindness; acid fumes are very harmful; store sulfuric acid in glass bottles with ground stoppers in wicker baskets with soft lining; Use only diluted acid. When diluting, the acid should be poured into the water in a thin stream, continuously stirring the solution. It is forbidden to pour water into acid, since when water combines with acid, a strong chemical reaction occurs, releasing a large amount of heat. Even when a small amount of water enters the acid, the water quickly heats up and turns into steam, which can lead to an explosion; — manual operations in which direct contact of the worker’s skin (washing, grinding in products, bottling, etc.) with dichloroethane (a flammable toxic liquid) or mixtures containing it are not allowed; — when heating the soldering iron, follow the general rules for safe handling of the heating source; — when working with blowtorches: check the serviceability of the lamp, pour fuel into the lamp no more than 75% of the capacity; It is unacceptable to add or pour fuel into a lamp that has not cooled down; Fill a kerosene lamp only with kerosene; use an electric soldering iron, the handle of which must be dry and non-conductive.
Rice. 2. Hard soldering: a - adjusting the surfaces of parts, b - lubricating the surfaces of parts with flux, c - inserting a copper plate, d - fixing the parts to be connected with a guide gasket, e - heating the parts
—-
There are several methods for brazing. These methods can be classified according to the way the metal is heated during the soldering process. Typically, brazing solders are divided into copper, copper-zinc, copper-nickel and silver. A separate group consists of aluminum solders. The most important brazing alloys are standardized.
PMTsZb solder is not used in mechanical engineering due to its low strength and brittleness. Solders PMTs48 and PMTs54 are rarely used due to insufficient ductility and low vibration resistance of the joints they solder. The most widely used solders are JI62 and JIOK 62-06-04, which provide strong solder joints. The tensile strength of JI62 solder is 30 kg/mm2 with an elongation of 35%.
The basis of most fluxes for hard soldering is Na2B407 borax, which crystallizes with ten parts of water into large transparent colorless crystals of Na2B407 • YN20. Crystalline borax begins to melt at 75 °C; as
As heating increases, it gradually loses water, strongly swelling and splashing, and turns into anhydrous salt - melted or burnt borax, melting at a temperature of 783 ° C. Borax in its molten state can be heated to high temperatures without noticeable evaporation; it is very fluid and energetically dissolves the oxides of many metals, especially copper oxides.
For soldering stainless steel, a mixture of equal parts of borax and boric acid, mixed with a saturated aqueous solution of zinc chloride to a paste, is used. When soldering gray malleable iron, strong oxidizing agents (potassium chlorate, manganese peroxide, iron oxide, etc.) are often added to the fluxes to burn off the graphite and increase the clean metal surface wetted by the solder.
Fluxes can be in powder or paste form. Fluxes are also used in the form of liquid solutions, for example a solution of borax in hot water. Sometimes it is advisable to use solder rods coated with flux. The fluxing effect can be exerted by the components of the solder itself. For example, phosphorus, oxidized into phosphoric anhydride, is a good flux for copper and copper alloys, reducing oxides and converting them into fusible phosphoric acid compounds. Therefore, phosphorous copper iripoi do not require fluxes for soldering copper alloys, which is very convenient in practice.
Rice. 1. Classification of brazing methods
Powdered fluxes can be sprinkled on the edges in a thin layer, often pre-heating the edges so that the flux particles melt, sticking to the metal, and are not blown away by the torch flame during soldering. The end of a solder rod, heated above the melting point of the flux, can also be dipped into powdered flux, which firmly adheres to the rod. Pastes and liquid solutions are applied with a brush or solder is dipped into them. You can make a paste of flux and powdered solder and apply it to the edge before soldering.
For soldering, preparatory work is important, often determining the quality of the connection. Three main forms of solder joints are widely used: lap, butt and miter joints (Fig. 239). The most common is the lap joint, which is easy to perform and very durable. By increasing the overlap of the lap joint, it is possible to increase its strength and, in most cases, achieve equal strength with the base metal. A butt joint has a better appearance and, with good solders and proper execution, can often provide sufficient strength (tensile strength can reach 40-45 kg/mm2). Butt joints are used in cases where doubling the thickness of the metal is undesirable. The miter joint, which requires advanced edge preparation, combines the advantages of butt and lap joints and provides a good appearance and no protruding edges. A miter connection makes it possible to achieve equal strength with the entire section by increasing the working area of the connection.
Of significant importance is the size of the gap between the edges being joined, which should be small both to improve the absorption of liquid solder by capillary forces and to increase the strength of the connection. For silver solders, a gap of 0.05-0.15 mm is recommended; For soldering with copper in shielding gas, gaps of 0.1-0.2 mm are recommended. Strict requirements regarding the size of the gap force fairly clean machining of surfaces, since rough processing, such as filing or sandblasting, can cause excessive solder consumption in the joint and a sharp drop in its strength.
For good solder wetting, the surface to be soldered must be immaculately clean. You can degrease with hot alkali, trichlorethylene or carbon tetrachloride. Oxides are removed by etching in acids, followed by thorough washing and drying.
Rice. 2. Forms of solder joints: 1 - lap joint; 2 - butt; z - “in the mouth”
Mechanical cleaning is carried out by wiping with a rag, fine sandpaper, grinding with fine-grained grinding wheels, brushes, etc. During assembly, flux is often first applied to the edges and solder is placed between the edges; in this case, solder is used in the form of foil or fine powder, or solder in the form of a wire or tape placed near the soldering site.
Before soldering, the assembled parts must be sufficiently firmly fastened with wire ties, pins, spot welding, etc., in order to eliminate the possibility of parts moving during heating and during the soldering process. The surface of products that should not be tinned is coated before soldering with a paste of chalk, clay, graphite or mixtures thereof, or moistened with a solution of chromic acid and similar substances that eliminate the adhesion of solder to the surface of the product.
—
Soldering of products with hard solders is used if permanent connections must have sufficient strength (temporary resistance 15-20 kgf/mm2).
Brazing alloys have a melting point above 450 °C.
To heat products when soldering with hard solders, various methods are used: with a gas flame (burner), in furnaces, in salt baths, with high-frequency currents, and on electric contact machines.
Where is it used?
Brazing is notable for the fact that when it is carried out, the area where the products are joined must be heated to temperatures of about 450 degrees or more.
Such solders are called refractory, and the joint obtained with their help retains its strength characteristics even with strong thermal heating.
Unlike hard soldering, soft soldering involves the use of low-temperature consumables, which provide reliable adhesion at significantly lower heat (about 200-300 ℃).
They are, as a rule, used when soldering products operated under normal temperature conditions, and do not guarantee the preservation of contact under high heat.
The capabilities of hard solders are widely used in those areas where it is necessary to obtain a seam whose strength properties occupy an intermediate position between welding and low-temperature soldering.
In this case, special attention is paid to preserving the structure of materials in the contact zone, which after processing should not lose their original characteristics.
Carbide compounds are most often in demand in the following situations:
- production of metal-cutting tools, cutters with carbide working inserts;
- in the manufacture of containers and vessels made from non-ferrous metals and stainless steel;
- in auto repair shops (when repairing radiators and individual transmission elements), as well as in those places where the use of welding is extremely undesirable;
- during installation and repair of tubes made of hard copper alloys installed in refrigeration and heat exchange equipment and operating under conditions of “critical” temperatures or high pressure;
- for reliable and durable connection of thin-walled objects and parts that experience increased loads and elastic deformations during operation.
The use of hard soldering technology ensures the necessary strength of the resulting connection and its resistance to overheating. In addition, carbide methods are used in the repair of copper or brass products that are exposed to high temperatures during operation.
Unlike the hard solders described above, the scope of application of soft soldering is limited to normal operating conditions. This method is most often used when it is necessary to obtain a reliable connection of products and parts made of low-melting materials that are not subject to excessive heating and deformation.
Widespread tin-lead soldering compounds are especially popular for “soft” jointing of parts.
How to choose a flux for soldering
Small elements are soldered with adjustable gas-air burners (this method is more suitable for jewelry). Larger parts are best soldered with acetylene. The same applies when choosing a flux for stainless steel, since this metal is very demanding on flux. Flux for stainless steel consists of 10% calcium fluoride, 20% boric acid, 70% borax .
For small stainless steel parts, you can prepare a flux composition that includes 50/50% boric acid and borax. This flux must be diluted in water, then applied to the part; when it dries, the solder will adhere perfectly to the metal surface.
That is, the soldering area is not etched, but only cleaned using sandpaper. Copper does not spread well over the surface of steel, so it is better to use L 63 brass. For better soldering, you can also use silver and brass to make solder from them.
Household use
Using the hard soldering technique at home requires the presence of a gas burner, through which a high degree of heating can be provided in the contact area.
In addition, you will need refractory solder itself, which melts at temperatures above 450 degrees, as well as a special active additive called flux.
Only if these requirements are met as a result of soldering work, it is possible to obtain a sufficiently reliable and hard soldered joint.
An example is the use of hard solder when soldering a bicycle frame, the restoration of which by other methods is not as effective and reliable.
Hard solders are in high demand when repairing various kitchen utensils and cookware made from carbide materials (brass or copper, for example).
Most often, samovars heated by coal or similar heating devices made of refractory metals are subject to restoration repairs.
Let us add to this that household brazing is widely in demand when carrying out repair work related to the restoration of individual units of refrigeration and heat exchange equipment.
In the latter case, soldering copper tubes using a gas torch will require hard brass solder, which allows you to obtain a strong and reliable connection suitable for use in critical conditions.
Let's look at the features of working with soldering compounds of varying degrees of refractoriness using the example of such a common operation as pipe sealing.
Soldering stainless steel at home
Perhaps everyone knows that a home handyman is constantly faced with everyday difficulties that he needs to eliminate on his own. But it often happens that you need to do the work associated with processing stainless steel yourself. Therefore, this will require certain skills, abilities and knowledge. You will also need to acquire some materials and tools. Here is a list of everything you need:
- Soldering acid;
- Electric soldering iron 100 Watt;
- Tin solder for joining metals;
- File or sandpaper;
- A tube;
- Metal cable.
Now that you have decided on the soldering tool, you need to know the steps to perform the actions:
- To process stainless steel, you must initially ensure that you have flux and a 100-watt electric soldering iron. You need to know that there is no point in choosing a more powerful soldering iron for processing stainless steel. The flux is ordinary soldering acid. Also, do not forget to always have tin-lead solder on hand.
- When all the necessary tools and materials are ready for soldering, you can begin work. First you need to clean the stainless steel joint: this can be done using sandpaper or a file. Upon completion of cleaning the work areas, it is necessary to apply soldering acid followed by further treatment. If the treatment does not work (the solder does not stick to the surface of the stainless steel), then you need to re-manipulate the soldering acid on a thoroughly heated surface, then perform the treatment again.
- In the case when you made a second attempt, and it was not successful, and the solder lags behind again, then the working surface of the stainless steel needs to be cleaned with a special brush, which you can make your own: you will need a piece of pipe with a cross-section of 5 mm, where you place thin wires pulled from a metal cable . Now, apply acid to the soldering area, and then bring the brush and soldering iron here at the same time. Then start working with two tools. It should be noted that this process is very helpful in removing the oxide film from the surface of stainless steel.
- When the parts have been tinned, begin soldering stainless steel using a soldering iron and flux.
Features of soldering pipe products
The order of sealing pipes with soft solders is determined by the following sequence of work operations:
- First, a joint with a socket is prepared from the pipes, and flux for soldering with soft solder is applied to the inner surface of the pipe.
- Then the same flux composition is applied to the outer part of the pipe being joined, after which the finished joint is heated to a temperature of about 300-400 degrees with a high-power soldering iron (at least one kilowatt).
- You can control the degree of heating of the docking unit by changing the color shade of the flux.
- After it has darkened, a wire rod of solder is introduced into the contact area (sometimes for better contact it is prepared in the form of small chips that fill all the joining gaps).
- Upon contact with the heated contact area, the solder melts and then, under the influence of flux, spreads over the entire area of the connecting seam.
High-temperature soldering using a solid composition differs from the procedures already described in the following ways.
Firstly, when implementing it, a flux of a completely different composition is applied to the joint, and secondly, the solder introduced into the soldering area must be made of refractory components.
And finally, to heat the contact zone with solid properties, special equipment is used (thermal oven, gas burner or induction heating device).
Both the processing of copper blanks and the soldering of steel at home require the use of a conventional gas burner, which is always available on the farm of any private owner.
Particular attention should be paid to the last stage of connecting pipe blanks, when, after softening the filler wire, one of the pipes rotates around its axis.
As a result of this operation, the solder, which has not yet hardened, is wound onto the joint area, followed by the formation of a reliable annular seam.
Methods for connecting parts
There are many ways to connect soldered parts to each other. This includes an overlap, a stepped seam, a corner connection, a comb, a butt and a flange connection.
The strength of soldered parts largely depends on the area of the surfaces being soldered. Therefore, the choice of method for joining workpieces must be approached carefully. The most durable joints are soldered joints with a comb and an overlap.
The T-joint is used quite rarely, since instead of it, a corner connection of the parts being soldered is most often used. In this case, the area of the connected surfaces increases noticeably.
For soldering sheet metal products, a lock connection is most often used. This connection ensures complete sealing of the seam.
After degreasing, the parts are connected to each other using one of the selected methods and securely fixed with clamps. It is very important that after fixing the correct position of the workpieces is not disturbed, since this guarantees a neat and high-quality joint.
Varieties
The main component of heat-resistant joints formed as a result of brazing is copper, from which almost all refractory consumables are made.
Pure copper is used extremely rarely as an adhesive component. As a rule, it is taken in combination with other metals (silver, zinc, silicon or tin).
Each of these additives makes the solder more refractory, and the resulting joint stronger and more durable.
Almost all of these impurities reduce the temperature at which the solid solder itself melts (for pure copper this figure is 1083 degrees).
For high-temperature processing of metals, as a rule, copper-zinc compounds are used, ideal for soldering bronze or copper parts (less commonly, steel).
However, they have one significant drawback, which is manifested in their poor protection from vibration and shock. In order to eliminate this drawback, the method of alloying with other metals is used, which significantly increases their strength characteristics.
Thus, hard brass solders can be considered as copper-zinc compositions that have undergone an alloying operation, thanks to which they are widely used in the manufacture of carbide cutters.
The main characteristics and areas of application of various types of solders can be found in the summary tables.
Use of tin-lead group alloys
The soldering process is the joining of several metallized parts together. In this case, the exposure temperature does not exceed the critical threshold at which destruction of parts or circuit boards occurs. The main objectives of using soldering products are to ensure the most even temperature viscosity, at which uniform spreading occurs over the surface.
Tin is used for soldering quite often; the material serves as a component of the largest number of solders. In its pure form, the metal is very expensive and is used for soldering important products and elements. Divided into categories with and without lead.
Lead solders
Various soldering materials are used using lead. The material is fusible, soft and easy to process. Easily dissolves in an alkaline environment and acidic impurities.
Lead solder
Products labeled PIC are considered the most popular in use. The percentage of elements allows you to work with different environments and materials. They differ in temperature and other parameters that are important for a reliable connection. Zinc, bismuth or antimony are added to lead compounds to provide protection against oxidation and other destructive factors.
Fluxes for refractory metals
The main component of flux additives used when working with hard solders are boron compounds, collectively called “borax” (Na2B4O7).
In order to increase the activity of fluxes of this class, a small amount of fluorine is added to them to form such active compounds as potassium fluoride and calcium.
To work with products made of copper and its hard alloys, it is advisable to use chemically pure borax, which is a universal flux composition that is optimally suited for high-temperature soldering conditions.
It should be noted that flux additives for soft and hard solders are available in a wide variety of forms (in the form of liquid, crystals or powder) and are often combined with solders.
This technique allows you to simplify the operation of their dosing and normalize the consumption of this component, which is important for high-quality soldering.
Soldering iron - the main tool
The main tool, without which it is impossible to connect parts and wires, is a soldering iron. There are many designs with different technical characteristics, so choosing the right product will not be an easy task for a beginner.
These tools are powerful and feature ceramic or spiral heaters. The former heat up very quickly, but require maximum care, since even minor impacts lead to breakage and failure. In the second case, soldering irons take longer to heat up, but are more practical and have a longer service life. Any of these modifications are used when solving the problem of how to solder with a soldering iron with tin and rosin.
Solders and fluxes
One of the main elements of electrical and radio installation work is soldering. The quality of installation is largely determined by the correct choice of the necessary solders and fluxes used when soldering wires, resistances, capacitors, etc.
To facilitate this choice, below is brief information about hard and light solders and fluxes, their use and their manufacture.
Soldering is the joining of hard metals using molten solder, which has a melting point lower than the melting point of the base metal.
The solder should dissolve the base metal well, spread easily over its surface, and well wet the entire soldering surface, which is ensured only if the wetted surface of the base metal is completely clean.
To remove oxides and contaminants from the surface of the metal being soldered, to protect it from oxidation and to provide better wetting with solder, chemicals called fluxes are used.
The melting point of fluxes is lower than the melting point of solder. There are two groups of fluxes: 1) chemically active, dissolving oxide films, and often the metal itself (hydrochloric acid, borax, ammonium chloride, zinc chloride) and 2) chemically passive, protecting only the surfaces to be soldered from oxidation (rosin, wax, stearin and etc.). .
Depending on the chemical composition and melting temperature of the solders, soldering is distinguished between hard and soft solders. Hard solders include solders with a melting point above 400°C, while light solders include solders with a melting point up to 400°C.
Basic materials used for soldering.
Tin is a soft, malleable metal with a silvery-white color. Specific gravity at a temperature of 20°C - 7.31. Melting point 231.9°C. It dissolves well in concentrated hydrochloric or sulfuric acid. Hydrogen sulfide has almost no effect on it. A valuable property of tin is its stability in many organic acids. At room temperature it is difficult to oxidize, but when exposed to temperatures below 18°C it can transform into a gray modification (“tin plague”). In places where gray tin particles appear, the metal is destroyed. The transition of white tin to gray accelerates sharply when the temperature drops to -50°C. For soldering it can be used both in pure form and in the form of alloys with other metals.
Lead is a bluish-gray metal, soft, easy to process, and can be cut with a knife. Specific gravity at a temperature of 20°C is 11.34. Melting point 327qC. In air it oxidizes only from the surface. It dissolves easily in alkalis, as well as in nitric and organic acids. Resistant to the effects of sulfuric acid and sulfuric acid compounds. Used for the manufacture of solders.
Cadmium is a silver-white metal, soft, ductile, and mechanically fragile. Specific gravity 8.6. Melting point 321°C. It is used both for anti-corrosion coatings and in alloys with lead, tin, bismuth for low-melting solders.
Antimony is a brittle, silvery-white metal. Specific gravity 6.68. Melting point 630.5°C. Does not oxidize in air. It is used in alloys with lead, tin, bismuth, cadmium for low-melting solders.
Bismuth is a brittle silver-gray metal. Specific gravity 9.82. Melting point 271°C. Dissolves in nitric and hot sulfuric acids. It is used in alloys with tin, lead, and cadmium to produce low-melting solders.
Zinc is a bluish-gray metal. When cold it is fragile. Specific gravity 7.1. Melting point 419°C. In dry air it oxidizes, in humid air it becomes covered with a film of oxide, which protects it from destruction. When combined with copper, it produces a number of durable alloys. Easily dissolves in weak acids. Used for the manufacture of hard solders and acid fluxes.
Copper is a reddish metal, malleable and soft. Specific gravity 8.6 - 8.9. Melting point 1083 C. Dissolves in sulfuric and nitric acids and ammonia. In dry air it is almost impossible to oxidize; in damp air it becomes covered with green oxide. Used for the manufacture of refractory solders and alloys.
Rosin is a product of processing the resin of coniferous trees. Lighter varieties of rosin (more thoroughly purified) are considered the best. The softening temperature of rosin is from 55 to 83°C. Used as a flux for soft soldering.
Soft solders.
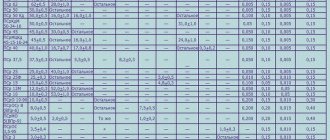
Soldering with soft solders has become widespread, especially during installation work. The most commonly used soft solders contain significant amounts of tin. In table Table 1 shows the compositions of some lead-tin solders.
Table 1
| Brand | Chemical composition in% | Temperature oC | ||||||
| tin | lead | antimony | no more impurities | |||||
| copper | bismuth | arsenic | Start | end | ||||
| POS-90 | 90 | 9,62 | 0,15 | 0,08 | 0.1 | 0,05 | 183 | 222 |
| POS-40 | 40 | 57,75 | 2,0 | 0,1 | 0,1 | 0,05 | 183 | 230 |
| POS-30 | 30 | 67,7 | 2,0 | 0,15 | 0,1 | 0,05 | 183 | 250 |
| POS-18 | 18 | 79,2 | 2,5 | 0,15 | 0,1 | 0,05 | 183 | 270 |
When choosing the type of solder, it is necessary to take into account its characteristics and use it depending on the purpose of the parts being soldered. When soldering parts that do not allow overheating, solders with a low melting point are used.
The most commonly used solder is POS-40 grade solder. It is used for soldering connecting wires, resistances, and capacitors. POS-30 solder is used for soldering shielding coatings, brass plates and other parts. Along with the use of standard grades, POS-60 solder (60% tin and 40% lead) is also used.
Soft solders are manufactured in the form of rods, ingots, wire (up to 3 mm in diameter) and tubes filled with flux. The technology of these solders without special impurities is simple and quite feasible in a workshop: lead is melted in a graphite or metal crucible and tin is added in small parts, the content of which is determined depending on the brand of solder. The liquid alloy is mixed, carbon deposits are removed from the surface and the molten solder is poured into wooden or steel molds. The addition of bismuth, cadmium and other additives is not necessary.
For soldering various parts that do not allow significant overheating, especially low-melting solders are used, which are obtained by adding bismuth and cadmium or one of these metals to lead-tin solders. In table Table 2 shows the compositions of some low-melting solders.
table 2
| Chemical composition in% | Melting point in °C | |||
| tin | lead | bismuth | cadmium | |
| 45 | 45 | 10 | _ | 1fi0 |
| 43 | 43 | 14 | __ | 155 |
| 40 | 40 | 21) | __ | 145 |
| 33 | 33 | 34 | __ | 124 |
| 15 | 32 | 53 | __ | 96 |
| 13 | 27 | 50 | 10 | 70 |
| 12,5 | 25 | 50 | 12,5 | 66 |
When using bismuth and cadmium solders, it should be taken into account that they are very brittle and create a less strong junction than lead-tin solders.
Hard solders.
Hard solders create high weld strength. In electrical and radio installation work they are used much less frequently than soft solders. In table Table 3 shows the compositions of some copper-zinc solders.
Table 3
| Brand | Chemical composition in% | Melting point in °C | |||||
| copper | zinc | no more impurities | |||||
| antimony | lead | tin | iron | ||||
| PMC-42 | 40—45 | rest | 0,1 | 0,5 | 1,6 | 0,5 | 830 |
| G1MTs-47 | 45—49 | 0,1 | 0,5 | 1,5 | 0,5 | 850 | |
| PMC-53 | 49-53 | 0,1 | 0,5 | 1,5 | 0,5 | 870 | |
The color of the solder changes depending on the zinc content. These solders are used for soldering bronze, brass, steel and other metals with a high melting point. PMC-42 solder is used when soldering brass containing 60-68% copper. PMC-52 solder is used for soldering copper and bronze. Copper-zinc solders are made by alloying copper and zinc in electric furnaces in a graphite crucible. As the copper melts, zinc is added to the crucible; after the zinc has melted, about 0.05% phosphorus copper is added. Molten solder is poured into molds. The melting temperature of the solder must be less than the melting temperature of the metal being soldered. In addition to the above-mentioned copper-zinc solders, silver solders are also used. The compositions of the latter are given in table. 4.
Table 4
| Brand | Chemical composition in% | Melting point in °C | ||||
| silver | copper | zinc | impurities no more | |||
| lead | Total | |||||
| PSR-10 | 9,7—10,3 | 52-54 | REST | 0,5 | 1,0 | 830 |
| PSR-12 | 11,7-12,3 | 35-37 | 0,5 | 1,0 | 785 | |
| PSR-25 | 24,7-25,3 | 39-41 | 0,5 | 1,0 | 765 | |
| PSR-45 | 44,5-45,5 | 20,5 —30,5 | 0,3 | 0,5 | 720 | |
| PSR-65 | 64,5-65,5 | 19,5 -—20,5 | 0,3 | 0,5 | 740 | |
| PSR-70 | 69,5-70,5 | 25,5— 26,5 | 0,3 | 0,5 | 780 | |
Silver solders have great strength; the seams soldered by them bend well and are easy to process. PSR-10 and PSR-12 solders are used for soldering brass containing at least 58% copper, PSR-25 and PSR-45 solders are used for soldering copper, bronze and brass, PSR-70 solder with the highest silver content is for soldering waveguides , volumetric contours, etc.
In addition to standard silver solders, others are used, the compositions of which are given in table. 5.
Table 5
| Chemical composition in% | Temperature melting in oC | ||||
| silver | copper | zinc | cadmium | phosphorus | |
| 20 | 45 | 30 | 5 | 780 | |
| 72 | 18 | __ | __ | __ | 780 |
| 15 | 80 | __ | __ | 5 | 640 |
| 50 | 15,5 | 16,5 | 18 | — | 630 |
The first of them is used for soldering copper, steel, nickel, the second, which has high conductivity, is used for soldering wires; the third can be used for soldering copper, but is not suitable for ferrous metals; The fourth solder has a special fusibility and is universal for soldering copper, its alloys, nickel, and steel.
In some cases, commercially pure copper with a melting point of 1083°C is used as solder.
Solders for soldering aluminum.
Soldering aluminum is very difficult due to its ability to easily oxidize in air. Recently, aluminum soldering using ultrasonic soldering irons has found application. In table Table 6 shows the compositions of some solders for soldering aluminum.
Table 6
| Chemical composition in% | Note | |||||
| tin | zinc | cadmium | aluminum | silicon | copper | |
| 55 | 25 | 20 | — | — | — | Soft solders |
| 40 | 25 | 20 | 15 | — | — | |
| 63 | 36 | — | 1 | — | — | |
| 45 | 50 | — | 5 | — | — | |
| 78—69 | 20-25 | 2-6 | — | — | ||
| 69,8—64,5 | 5,2-6,5 | 25-29 | Brazing alloys with a melting point of 525°C | |||
When soldering aluminum, organic substances are used as fluxes: rosin, stearin, etc.
The last solder (hard) is used with a complex flux, which includes: lithium chloride (25-30%), potassium fluoride (8-12%), zinc chloride (8-15%), potassium chloride (59-43%) . The melting point of the flux is about 450°C.
Fluxes.
Good wetting of solder joints and the formation of strong seams largely depends on the quality of the flux. At the soldering temperature, the flux should melt and spread in an even layer, and at the moment of soldering it should float to the outer surface of the solder. The melting point of the flux should be slightly lower than the melting temperature of the solder used.
Chemically active fluxes (acid) are fluxes that in most cases contain free hydrochloric acid. A significant disadvantage of acid fluxes is the intense formation of corrosion of solder seams.
Chemically active fluxes primarily include hydrochloric acid, which is used for soldering steel parts with soft solders. The acid remaining on the surface of the metal after soldering dissolves it and causes corrosion. After soldering, the products must be rinsed with hot running water. The use of hydrochloric acid when soldering radio equipment is prohibited, since during operation it is possible to break the electrical contacts at the soldering points. Please note that hydrochloric acid causes burns if it comes into contact with the body.
Zinc chloride (etching acid), depending on the soldering conditions, is used in the form of a powder or solution. Used for soldering brass, copper and steel. To prepare the flux, it is necessary to dissolve one part by weight of zinc in five parts by weight of 50% hydrochloric acid in a lead or glass container. A sign of the formation of zinc chloride is the cessation of the release of hydrogen bubbles. Due to the fact that there is always a small amount of free acid in the solution, corrosion occurs at the soldering joints, so after soldering the joint must be thoroughly washed in running hot water. Soldering with zinc chloride should not be carried out in the room where the radio equipment is located. It is also prohibited to use zinc chloride for soldering electrical and radio equipment. Zinc chloride should be stored in a glass container with a tightly closed glass stopper.
Borax (aqueous sodium salt of pyroboric acid) is used as a flux when soldering with brass and silver solders. Easily dissolves in water. When heated, it turns into a glassy mass. Melting point 741°C. Salts formed during brown soldering must be removed by mechanical cleaning. Borax powder should be stored in hermetically sealed glass jars.
Ammonia (ammonium chloride) is used in powder form to clean the working surface of a soldering iron before tinning.
Chemically passive fluxes (acid-free).
Acid-free fluxes include various organic substances: rosin, fats, oils and glycerin. Rosin (in dry form or a solution in alcohol) is most widely used in electrical and radio installation work. The most valuable property of rosin as a flux is that its residues after soldering do not cause corrosion of metals. Rosin has neither reducing nor dissolving properties. It serves solely to protect the soldering area from oxidation. To prepare alcohol-rosin flux, take one part by weight of crushed rosin, which is dissolved in six parts by weight of alcohol. After the rosin has completely dissolved, the flux is considered ready. When using rosin, soldering areas must be thoroughly cleaned of oxides. Often, for soldering with rosin, parts must be pre-tinned.
Stearin is non-corrosive. Used for soldering lead sheaths of cables, couplings, etc. with especially soft solders. Melting point is about 50°C.
Recently, the LTI group of fluxes used for soldering metals with soft solders has become widely used. In terms of their anti-corrosion properties, LTI fluxes are not inferior to acid-free ones, but at the same time, they can be used to solder metals that previously could not be soldered, for example, parts with galvanic coatings. LTI fluxes can also be used for soldering iron and its alloys (including stainless steel), copper and its alloys and metals with high resistivity (see Table 7).
Table 7
| Name | In weight proportions | ||
| LTI-1 | LTI-115 | LTI-120 | |
| Raw alcohol or rectified alcohol | 67-73 | 63-74 | 63-74 |
| Rosin | 20-25 | 20-25 | 20-25 |
| Aniline hydrochloride | 3-7 | — | — |
| Metaphenylenediamine | — | 3-5 | — |
| Diethylamine hydrochloride | — | — | 3-5 |
| Triethanolamine | 1-2 | 1-2 | 1-2 |
When soldering with LTI flux, it is enough to clean the soldering areas only from oils, rust and other contaminants. When soldering galvanized parts, you should not remove zinc from the soldering area. Before soldering parts with scale, the latter must be removed by etching in acids. Pre-etching of brass is not required. Flux is applied to the joint using a brush, which can be done in advance. Flux should be stored in glass or ceramic containers. When soldering parts with complex profiles, you can use solder paste with the addition of LTI-120 flux. It consists of 70-80 g of petroleum jelly, 20-25 g of rosin and 50-70 ml of LTI-120 flux.
But fluxes LTI-1 and LTI-115 have one big drawback: after soldering, dark spots remain, and intensive ventilation is required when working with them. Flux LTI-120 does not leave dark spots after soldering and does not require intensive ventilation, so its use is much wider. Usually, flux residues after soldering do not need to be removed. But if the product will be used in severe corrosive conditions, then after soldering, flux residues are removed using ends moistened with alcohol or acetone. The production of flux is technologically simple: alcohol is poured into a clean wooden or glass container, crushed rosin is poured until a homogeneous solution is obtained, then triethanolamine is added, and then active additives. After loading all the components, the mixture is stirred for 20-25 minutes. The prepared flux must be checked for a neutral reaction with litmus or methyl orange. The shelf life of the flux is no more than 6 months.
What is soldering
Soldering is a method of joining metal parts using a lower melting metal. The process itself is the penetration of one substance into another at high temperature (180-250 degrees).
Important! The main thing is high-quality heating of the parts to be soldered, followed by fixing them with solder.
Having mastered the soldering technique, the master will be able to connect any parts
Preparing the soldering iron and parts
If the soldering iron is new, you need to turn it on for a few minutes and let it run “idle”. This is necessary for the factory grease to burn out. With such preparation, the soldering iron may smoke, so do not be alarmed.
Usually soldering irons are sold with a tinned tip (coated with a layer of tin). If the tip is uncovered, it needs to be lightly sanded with sandpaper, heated, then dipped in flux and coated with solder.
Sometimes even a tinned tip needs to be lightly cleaned before soldering. During operation, the tip becomes covered with an oxide film, which impairs the adhesion of solder. To do this, lightly clean it with sandpaper or a cloth, depending on the degree of contamination.
To prepare the parts you need:
- Remove the insulation (if wires are soldered).
- Degrease.
- Coat the part with flux.
- Tin with a soldering iron.
Then you can proceed directly to soldering. It is usually necessary to tin both parts. To remove oxides, you can use a soldering iron tip, sandpaper or a sharp knife.
Soldering technology
There are two types of technologies, namely using flux or rosin. It is worth noting that the choice of technology for carrying out work depends entirely on the master, here everyone proceeds from their own experience.
Soldering with rosin
Soldering with a rosin tool is much more difficult than using flux. However, with mastery of the technique, anyone can complete 90 percent of the tasks.
A striking example will be the technique of how to properly solder wires with a soldering iron. Initially, you should warm it up: apply the tip of the tool flat. Then lower the wire with the pressed tip into the rosin. After completing this procedure, he becomes prepared. Then, with the tip of the device, you need to take a small part of the solder and apply it to the wire.
The tip of the device must be cleaned with a metal sponge and, touching the rosin, run the device over the board, leaving a thin layer of rosin on the surface. After performing these manipulations, the surfaces become prepared. Finally, you should press the tip to the wire with a thin layer of solder and “walk” along the soldering area.
Important! If the work is done correctly, the surface will shine and the connection will have high strength.
Soldering with flux
To solder, you will need to dip a brush into the flux and move it to the soldering site. Then, apply solder and begin the process.
It would seem that the procedure is simple and even a person with no experience can perform it. However, it is not. Working with acid has a lot of difficulties:
- You should select your own flux for each material, since they are not interchangeable and can often have the opposite effect;
- It is strictly forbidden to use overly active fluxes on microcircuits;
- At the end of using the device, it is important to remove any remaining flux, otherwise it will contribute to the decomposition of the metal.