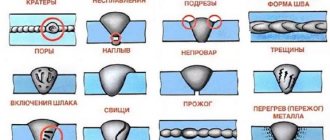
Chemical resistance of aluminum and its alloys.
The standard electrode potential of aluminum is -1.66V, i.e. it is a fairly active metal. However, due to its tendency to passivate, aluminum can be resistant in many environments.
In its normal state, the surface of aluminum is covered with a layer of oxide with a thickness of 5 to 100 nanometers. The film is firmly adhered to the metal and covers it with a continuous layer. A film on aluminum is formed at pH=3-9. The corrosion resistance of aluminum is higher for the most pure aluminum (AB1 and AB2) with an aluminum content of 99.9-99.85%, respectively, and lower for technically pure A00 and A0 with an aluminum content of 99.7-99.6, respectively. Duralumin (duralumin, 2-7% copper) has low corrosion resistance. Silumin casting alloys (0.8-13% silicon) hold up well in oxidizing environments.
Aluminum struts:
- In atmospheric conditions;
- In environments containing H2S, SO2, NH3;
- In water when heated;
- In solutions of salts with oxidizing properties - chromate, nitrate;
- In concentrated solutions of nitric and sulfuric acids (have oxidizing properties);
- In dilute sulfuric acid at 20°C;
- In oleum up to 200o C;
- In phosphoric acid at room temperature;
- In acetic acid with a concentration of 1-99% by weight at temperatures up to 65o C;
- In boiling acetic acid only at concentrations of 98-98.8% wt;
- In formaldehyde;
- In dry hydrogen chloride.
Aluminum is unstable:
- In neutral solutions of salts containing halides - fluorides, chlorides, bromides, iodides;
- In sulfuric acid of medium concentration;
- In boiling acetic acid up to 98% mass and above 98.8% mass;
- In droplet liquid and vapor mercury (corrosion of aluminum in acetic acid begins in the presence of mercury 0.000004% wt;
- In alkalis (with hydrogen depolarization);
- In hydrofluoric acid;
- In contact with copper, iron and their alloys.
Resistance of coatings to corrosion under the influence of temperature changes and high humidity
To estimate a parameter with these conditions, methods 12-14 are used
Method 12
To assess corrosion resistance using this method, at least 15 cycles are carried out.
At the beginning of each cycle, samples are kept in a moisture chamber for 6 hours at a temperature of 40 degrees and a humidity of 97 percent. After turning off the heating, they are kept for another 2 hours. Next, the products are tested in a cold chamber for 3 hours at a temperature of 42-48°C. After this, the samples are subjected to high temperatures in thermal chambers (at 60 degrees) for 7 hours. At the end of the cycle, they are kept at 15-30 degrees for 6 hours (humidity up to 80%). At the end of the last cycle an assessment is made.
Method 13
To carry out testing using the thirteenth method, the test objects are first placed in a moisture chamber for 2 hours. It is pre-set at 40 degrees Celsius and humidity at 97%. After the heating is turned off, they are kept for the same amount of time.
The next chamber is a chamber with low temperatures (-30°C). The tested object is kept in it for 6 hours. After this, the products are subjected to elevated temperatures, namely 60 degrees, in a heat chamber. The duration of exposure is 5 hours. After this, the objects are mixed back into the cold chamber (-60) and kept for another 3 hours.
Finally, the product is kept in normal conditions for 6 hours (15-30 degrees, 80% air humidity).
All these procedures constitute one cycle. For an objective assessment of the parameter, 15 or more cycles are carried out.
Method 14
The number of cycles for method 14 depends on conditions T3 and T2, but in any case their number is more than 10.
The tested product is kept first for 10 hours in a moisture chamber at 55 degrees C (97% humidity), then for another 2 hours with the heating turned off. Afterwards they are placed in a heat chamber (60°C) for 10 hours. Then take it out and keep it for 2 hours at 15-30°C (80% air humidity).
Afterwards, an assessment is made based on the data received.
Chemical resistance of copper and its alloys.
The standard copper potential is +0.52/0.337V for the reduction of cuprous and divalent copper, respectively. Typically, during corrosion, copper goes into solution in its divalent form. The standard potential of copper in a solution of 3% sodium chloride is +0.05V, and in a solution of 1N hydrochloric acid is +0.15V. Therefore, copper under normal conditions does not displace hydrogen from solutions, i.e. cannot corrode with hydrogen depolarization. The ability to passivate copper is weakly expressed. Resistance to gas corrosion of copper increases when alloyed with beryllium, magnesium and aluminum.
Brass is an alloy of copper and zinc. The introduction of aluminum, manganese, and nickel into brass increases the resistance of the alloy to atmospheric corrosion, and silicon to sea water.
Copper is resistant:
- In saline solutions;
- In dilute non-oxidizing acids;
- In formaldehyde.
Copper is unstable:
- In solutions where it can form complexes (cyanides, ammonia);
- In solutions of oxidizing agents - nitric acid, hydrogen peroxide;
- In the presence of dissolved oxygen (especially when blowing it through the solution);
- In chromic acid;
- In formic acid;
- In sulfides, polysulfides, sulfur dioxide.
Method 9
Method 9 is used to evaluate resistance to corrosion when exposed to elevated temperature, humidity, salt fog and solar radiation . For evaluation, samples are subjected to different climatic conditions, repeated for 20 or more cycles.
Each cycle begins with a moisture chamber, set between 53-57oC and 97 percent humidity. After holding for 5 hours, the objects are moved to a salt fog chamber, where they are kept for 3 hours. After this, the objects are returned to the moisture chamber, where they are subjected to the same conditions for the same amount of time. In the light chamber with dir. 4-16 they are kept for 10 hours. After which it is stored in air for 1 hour at no less than 15°C and no more than 30°C (humidity up to 80 percent).
The assessment is carried out at the end of the last cycle based on clause 6.12.6-10 of GOST 9.401-2018.
Chemical resistance of nickel and its alloys.
The standard potential of nickel is -0.25V. Nickel corrosion mainly occurs with oxygen depolarization.
Nickel resistant:
- In non-oxidizing dilute acids (hydrochloric acid up to 15%, sulfuric acid up to 70%);
- In a number of organic acids, alcohols;
- In any alkalis at any temperature.
Nickel is unstable:
- In the presence of iron (III) chloride, copper (II) chloride, mercuric (II) chloride, silver nitrate, NaClO;
- In oxidizing acids (for example, nitric acid);
- In concentrated non-oxidizing acids.
Chemical resistance of tin and its alloy with bismuth.
The standard potential of tin is -0.136V. Pure tin is compact at temperatures above +13o C (in the form of white tin). Below this temperature, especially at -48o C, tin actively transforms into the allotropic modification “gray tin”, which has a powdery structure. To eliminate this phenomenon, tin is doped, for example, with a small amount of bismuth (0.5-2%). Tin is weakly passivated.
Tin is stable:
- In natural waters;
- In solutions of neutral salts;
- In food environments;
- In dilute solutions of sulfuric and hydrochloric acids;
- In organic acids.
Definition of corrosion
Metal materials under chemical or electrochemical influence of the environment are subject to destruction, which is called corrosion.
Corrosion of metals is caused by redox reactions, as a result of which metals become oxidized and lose their properties, which renders metal materials unusable.
There are 3 signs that characterize corrosion:
- Corrosion is, from a chemical point of view, a redox process.
- Corrosion is a spontaneous process that occurs due to the instability of the thermodynamic system metal - environmental components.
- Corrosion is a process that develops mainly on the surface of the metal. However, it is possible that corrosion can penetrate deep into the metal.
Chemical resistance of lead.
The standard lead potential is -0.126V. The corrosion resistance of lead is largely determined by the stability of its corrosion products.
Lead resistant:
- In sulfuric acid and sulfates;
- In phosphoric acid and phosphates;
- In hydrochloric acid up to 10%;
- In hard waters with calcium sulfate;
- In silicic acid;
- In industrial atmospheres with hydrogen sulfide, sulfur dioxide and sulfuric acid.
Lead is unstable:
- In nitric acid;
- In acetic acid;
- In alkalis;
- In sulfuric acid above 96% and oleum;
- In hot sulfuric acid up to 80%;
- In hydrochloric acid over 10%;
- In groundwater with organic acids;
- In underground waters saturated with carbon dioxide.
Method 1
The method makes it possible to evaluate the resistance of coatings to corrosion when exposed to changes in temperature values, high air humidity and destructive solar exposure over a short period of time . Certain conditions are established for testing. The temperature is 40°C and the air humidity is 97%.
The test consists of 5 cycles. Where each cycle begins with keeping the sample under the specified conditions for 1 hour. After this, the heating is turned off and the product is left for another 2 hours. Next they are placed in a light chamber with dir. 3-17 for another 2 hours. As a final step, the samples are kept in air for 19 hours under conditions with a temperature range of 15-30°C and air humidity of up to 80%.
After 5 cycles, samples are evaluated.
6. Chemical resistance of zinc.
The standard potential of zinc is -0.76V. Zinc can corrode with both hydrogen and oxygen depolarization. It is rarely used in its pure form, mainly in chromated or chromium-plated form, as well as in passivated form using chromium-free passivators.
Zinc stable:
- In fresh water up to 55°C;
- In clean and sea dry atmospheres.
Zinc is unstable:
- In acidic environments (pH below 7);
- In alkaline environments (at pH above 12);
- In industrial environments containing SO2, SO3, HCl;
- In sea water and humid marine atmosphere.
Methods of protection against metal corrosion
The main method of protecting metal from corrosion is the creation of protective coatings - metallic, non-metallic or chemical.
Metal coatings
A metal coating is applied to the metal that needs to be protected from corrosion with a layer of another metal that is resistant to corrosion under the same conditions. If the metal coating is made of a metal with a more negative potential (more active) than the one being protected, then it is called an anodic coating . If the metal coating is made of a metal with a more positive potential (less active) than the one being protected, then it is called a cathodic coating .
For example, when applying a layer of zinc to iron, if the integrity of the coating is compromised, the zinc acts as an anode and will be destroyed, while the iron is protected until all the zinc is used up. The zinc coating in this case is anodic .
The cathode coating to protect the iron may, for example, be copper or nickel. If the integrity of such a coating is violated, the protected metal is destroyed.
Non-metallic coatings
Such coatings can be inorganic (cement mortar, glassy mass) and organic (high molecular weight compounds, varnishes, paints, bitumen).
Chemical coatings
In this case, the protected metal is subjected to chemical treatment in order to form a corrosion-resistant film of its compound on the surface. These include:
oxidation – production of stable oxide films (Al2O3, ZnO, etc.);
phosphating – obtaining a protective film of phosphates (Fe3(PO4)2, Mn3(PO4)2);
nitriding – the surface of the metal (steel) is saturated with nitrogen;
steel bluing - the metal surface interacts with organic substances;
carburization – obtaining on the surface of a metal its connection with carbon.
Changes in the composition of technical metal and corrosive environment
Changing the composition of the technical metal also helps to increase the metal's resistance to corrosion. In this case, compounds are introduced into the metal that increase its corrosion resistance.
Changing the composition of the corrosive environment (introducing corrosion inhibitors or removing impurities from the environment) is also a means of protecting the metal from corrosion.
Electrochemical protection
Electrochemical protection is based on connecting the protected structure to the cathode of an external direct current source, as a result of which it becomes the cathode. The anode is scrap metal, which, when destroyed, protects the structure from corrosion.
Protective protection - one of the types of electrochemical protection - is as follows.
Plates of a more active metal, called protector . The protector - a metal with a more negative potential - is the anode, and the protected structure is the cathode. The connection of the protector and the protected structure with a current conductor leads to the destruction of the protector.
Examples of problems with solutions for determining the protective properties of oxide films, determining the corrosion resistance of metals, as well as equations for reactions occurring during electrochemical corrosion of metals are given in the section Problems for the section Corrosion of metals
Categories Corrosion of metals, GENERAL CHEMISTRY
Chemical resistance of cadmium.
The standard potential of cadmium is -0.4V. Cadmium has a low passivation ability. Corrosion behavior is similar to zinc, however, as the pH decreases, the corrosion rate decreases. Cadmium is more stable in acidic and neutral environments than zinc. In alkaline environments, cadmium is quite stable. The most important thing is that cadmium, unlike zinc, is stable in sea water and this determines its main use. In the presence of SO2 and SO3, cadmium quickly corrodes. Like zinc, cadmium coatings are used in chromated form.
2507 Super Duplex Stainless Steel Alloy
Stainless steel
Duplex stainless steel has a two-phase microstructure consisting of austenite and ferrite grains. This structure gives these materials a combination of attractive properties, including strength, ductility and corrosion resistance.
Super duplex ferritic-austenitic stainless steel 2507 is excellent for use in highly corrosive environments. Its composition includes nickel, molybdenum, chromium, nitrogen and manganese, which provides excellent resistance to solid, pitting and crevice corrosion, stress corrosion, and stress cracking while maintaining weldability.
- Increased yield strength and tensile strength at increased pressure ratings.
- Compared to 316/316L steel tubing of the same outside diameter and pressure rating, the thinner wall thickness allows for increased fluid flow.
- Weldability.
- Applications with temperatures up to 482°F (250°C).
- Higher thermal conductivity/lower coefficient of thermal expansion compared to 316 stainless steel.
- Suitable for use in sour gas environments (NACE MR0175/ISO 15156)
- Swagelok Alloy 2507 products are offered in rods and forgings that meet the NORSOK M-650 steel supplier standard.
Alloy 2507's mechanical properties make it an excellent choice for high-pressure marine and subsea applications where corrosion, high flow rates and weight must be considered.
To combat:
continuous corrosion; local corrosion; stress corrosion cracking
Chemical resistance of titanium.
The standard potential of titanium is -1.63/-1.21V for the divalent and trivalent forms, respectively. Titanium is prone to passivation.
Titanium resistant:
- In oxidizing environments (including chromates, permanganates, hydrogen peroxide, oxygen, nitric acid);
- In the presence of chloride ions;
- In aqua regia;
- In iron (III) chloride up to 30% and up to 100o C;
- In copper (II) chloride up to 20% and up to 100o C;
- In mercury (II) chloride of all concentrations up to 100 ° C;
- In aluminum chloride up to 25% and up to 60o C;
- In sodium chloride of all concentrations up to 100o C;
- In sodium hypochlorite solution up to 100o C;
- In chlorine water;
- In gaseous chloride up to 75o C;
- In hydrochloric acid no more than 3% at 60o C;
- In hydrochloric acid no more than 0.5% at 100o C;
- In phosphoric acid up to 30 no higher than 35o C;
- In phosphoric acid up to 3% at 100o C;
- In a humid chlorine atmosphere (in the presence of above 0.005% moisture);
- In alkalis up to 20%;
- In many organic environments.
Titan is unstable:
- In hydrochloric acid above 3% at 60o C;
- In hydrochloric acid more than 0.5% at 100o C;
- The maximum dissolution of titanium in sulfuric acid is observed at 40% and 75%;
- In an atmosphere of absolutely dry chlorine;
- In alkalis above 20%.
About the corrosion resistance of tool steel
Iron and steel are susceptible to corrosion. The more carbon in steel, the more likely it is to corrode. You can increase the corrosion resistance of steel, that is, corrosion resistance, in three ways:
1st way.
The corrosion resistance of steels is determined by the presence of alloying elements that can make the steel more noble, that is, located to the right of iron in the series of electrochemical activity of metals:
Li→Rb→K→Ba→Sr→Ca→Na→Mg→Al→Mn→Cr→Zn→Fe →Cd→Co→Ni→Sn→Pb→H→Sb→Bi→Cu→Hg→Ag→Pd→Pt→Au
there are very few such elements, and their contribution to increasing corrosion resistance is not very large, since the introduction of these elements in quantities sufficient to increase corrosion resistance into the steel composition turns out to be impossible or difficult. The presence of elements such as Ni, Co, Mo, W, Cu in the steel composition indicates increased corrosion resistance, however, even high-speed steels containing large amounts of tungsten and molybdenum are not corrosion resistant.
2nd way.
Alloying elements in steel capable of forming a strong oxide film on the surface of the steel. Ironically, these elements are in the series of electrochemical activity to the left of iron, that is, more active. These elements could be titanium, chromium, aluminum, silicon, but only chromium can be introduced in quantities at which the steel becomes “stainless,” that is, covered with a continuous film of chromium oxide. Chromium oxide is extremely chemically resistant and its film protects steel from exposure to aggressive environments. In order for the oxide film to be continuous, free chromium in the steel must be more than 13%. Free means capable of combining with oxygen in the air and not chemically bonded with other atoms in the steel. The maximum corrosion resistance of chromium steels is achieved after hardening, since it is hardening that makes the chromium in the steel free.
3rd way.
Carbon reduction. Because the presence of carbon increases strength and wear resistance, the third route is rarely used in tool steels. There are only a few grades of tool steels in which carbon is replaced by nitrogen. The maximum corrosion resistance is achieved by steels whose corrosion resistance is increased in three ways; most often, these steels contain very little carbon, a lot of chromium and nickel, and also contain a certain amount of titanium or molybdenum. These are the so-called “food grade stainless steel” in common parlance, used in the food and chemical industries. However, the presence of nickel in large quantities makes the steel unable to accept hardening. Steels of type 12Х18Н10Т, 12Х23Н18 are used in knifemaking for the manufacture of knife accessories, for example, screws, lanyard tubes. Steels for cutting blade tools have less corrosion resistance because they do not contain significant amounts of nickel and most often contain a lot of carbon.
Based on corrosion resistance, steels used for the manufacture of cutting blade tools can be divided into several groups.
Group-1:
Actively corrosive or non-corrosion-resistant steels. These are steels that do not contain alloying elements (carbon), as well as steels that are alloyed and contain alloying elements in amounts up to 20%, but the chromium content does not exceed 6%. Such steels require careful care when using knives, and possibly conservation during storage. Corrosion with the formation of rust occurs on such steels within minutes. It should be noted that many representatives of the first group are champions in maintaining the sharpness of the cutting edge.
Group-2:
Conditionally corrosion-resistant steels, often not actively forming rust, but corroding with the formation of patina (darkening). These steels can be called steels with increased corrosion resistance. For corrosion to occur with the formation of rust, such steels must be stored for hours or tens of hours in a humid environment. These are steels containing alloying elements in quantities of more than 10%, and the chromium content, as the most important indicator of corrosion resistance, ranges from 6-12%. Knives made from such steels require wiping after use.
Group-3:
Corrosion-resistant steels that do not form rust and are almost non-corrosive in a humid atmosphere. These steels always contain at least 13% chromium, but a high chromium content does not guarantee corrosion resistance. The maximum corrosion resistance of chromium tool steels occurs in a low-temper quenched state. In order for corrosion of properly heat-treated corrosion-resistant steels to occur with the formation of rust, they must be stored for several days, and sometimes months, in a humid environment in the presence of organic acids, enzymes, salts, and active oxidizing reagents. For example, pitting corrosion is possible when stored in a damp sheath, because leather may contain both organic substances and mineral oxidizing agents used for tanning. Despite the fact that these steels are colloquially referred to as “stainless steels,” this is not entirely true: there are no absolutely stainless tool steels!
The term "stainless" is usually used for marketing purposes. Tools from this group of steels most often do not require special care, however, for reasons of safety of both the tool and often health, do not forget to wash and wipe the knives dry after use. Even the most corrosion-resistant nitrogen steels such as Cronidur-30 have a corrosion rate tens of times lower than that of their closest neighbors, but this corrosion rate is not zero.
Burov S.V.