01/26/2022 Author: VT-METALL
From this material you will learn
:
- Concept of precision alloys
- Brief characteristics of iron and nickel
- The history of the discovery of an alloy of iron and nickel
- Alloy manufacturing process
- Structure of the resulting alloy
- Physical and chemical properties of the alloy
- Areas of application of Invar
Invar (iron-nickel alloy)
is simply necessary for creating high-precision mechanical systems, one of the requirements for which is maintaining stable dimensions when temperature changes. None of the natural materials has such properties.
This is why the precision alloy Invar is so in demand by enterprises today. It is used in a variety of areas of human activity, for example, in instrument making and consumer electronics. And an adequate replacement has not yet been found.
Fechral and nichrome: comparison of alloys
The alloys fechral and nichrome are quite similar, but according to many craftsmen, preference should be given to nichrome. Fechral and nichrome belong to the group of precision alloys, that is, they have precisely defined characteristics. Although both of the above alloys have significant value for industry due to their inherent qualities, there are differences that affect the “fulfillment” of the tasks assigned to them.
So, for example, fechral is considered more fragile than nichrome, since it does not contain nickel (Ni), which tends to increase the strength and survivability of the alloy in watches.
Composition and structure
The structure of the aluminum-iron alloy is a supersaturated solution of Al in α-Fe with an ordering of the FeAl structure (type B2) and the presence of Fe3AlCx inclusions. Properties are determined by alpha phase ordering and supersaturation. To form a homogeneous composition, annealing is necessary at a temperature above the ordering of the composition, followed by controlled cooling.
When the amount of Al is 8–14%, a columnar matrix structure is formed. During the annealing process, the structure becomes slightly ordered: inclusions up to 150 µm long are located along the grain boundaries. The release of inclusions occurs during cooling from the solid phase.
The metastable state of the phase is determined by the number of inclusions. Annealing allows them to be reduced to 2%. The more aluminum in the composition, the more inhomogeneous regions are created, as a result of which the microhardness of the matrix decreases to 0.4 GPa and the wear resistance of the sample decreases.
With increasing annealing speed during water or air cooling, the number of carbide inclusions decreases.
A 14-20% aluminum-iron alloy also has a matrix structure, but the carbide phase is depleted in Al and the FeAl structure is not ordered. When annealing in air, the number of carbide inclusions increases, due to which the wear resistance and strength properties increase. If cooling is carried out in water, then this effect is not observed and the sample turns out to be fragile.
When the Al content in the alloy is increased from 20 to 30%, the carbide phase becomes smaller; when the samples are cooled, this phase is absent from the structure or no more than 3%. Due to the large amount of aluminum, the sample acquires high strength and ductility. Air cooling after annealing stimulates the formation of hard wear-resistant phases.
An increase in the aluminum content in the melt causes the formation of the Fe4Al13 intermetallic compound, which is not eliminated after annealing, and the sample becomes unsuitable for any practical use.
To improve the properties of the melt, the following alloying elements are introduced into the composition:
- 0.1–10% Cr;
- 0.1–0.2% Nb;
- 0.1–2.0% Si;
- 0.1–5% B;
- from 50 to 200 mg/kg Zr.
Carbon content - from 100 to 500 mg/kg.
Melting temperature
Fechral - an alloy of iron, chromium and aluminum
Fechral is a precision alloy based on iron (Fe). It also contains chromium (Cr) and aluminum (Al). In addition, some other elements are present in a small percentage.
This alloy can have several modified compositions and, accordingly, grades. The most common are fechral X23YU5T, X27YU5T, and X15YU5.
As mentioned earlier, the most significant component is iron (Fe). It is about 70% in Kh23Iu5T and 66% in Kh27Iu5T.
The price of fechral is lower than nichrome, precisely due to the high iron content. The element (Fe) degrades some characteristics of the alloy.
The next component is chromium (Cr). Its content is 23-25% and 27-28%, respectively.
Aluminum (Al) in X23Iu5T and X27Iu5T is contained in almost equal quantities - from 5% to 6%.
The X15Yu5 grade of fechral is characterized by a high iron (Fe) content, close to 80%, and a reduced chromium (Cr) content - from 13% to 15%.
Thanks to this, the alloy gains strength, but not as much as that of nichrome. The percentage of aluminum does not differ much from other brands, it is no more than 6%, no less than 4%.
All grades of fechral also contain nickel (Ni), titanium (Ti), silicon (Si), manganese (Mn) and carbon (C). Their share is up to 1%. It also contains calcium (Ca), cerium (Ce), phosphorus (P) and sulfur (S) in very small quantities.
Alloy manufacturing process
In its external characteristics and to the touch, Invar is similar to steel. And this is quite logical, because we are talking about an alloy of ferrous metals, where iron plays the role of the main component. Invar contains between 0.01–0.1% carbon, but this figure is usually at the lower limit, since we are talking about a very pure metal.
To produce this alloy of iron and nickel, the galvanic method is used. A comparison of the properties of the metals we are interested in showed that creating invar is not so difficult. But the problem turned out to be that as a result of the reaction, iron passes from a divalent to a trivalent state. This side effect caused the main difficulties in the manufacture of such an important alloy, leading to a reduced yield of the material and a decrease in its physical characteristics.
It was possible to reduce the negative effect due to a complex of additives containing organic substances, acids, and amines. In this way, compounds of low solubility with ferric iron were obtained, improving the quality of the material.
The method of effective diffusion of an electrolytic solution allows you to get rid of sediment. The latter contains iron sulfate, boric acid, saccharin, nickel sulfate and sodium sulfate.
When using nickel and iron plates, it is important to consider the plate size. In some cases, compounds are produced using electric ovens.
Pros and cons of fechral
The presented alloy, the main components of which are iron, chromium and aluminum, has its advantages and disadvantages.
Advantages:
- low electrical conductivity;
- cheaper than nichrome;
- has a high operating temperature (up to 1400 °C);
- good heat resistance.
Flaws:
- too fragile and short-lived;
- highly magnetic (caused by high iron content);
- susceptible to oxidation;
- Winding is possible only in a heated state.
Introduction.
The issue of corrosion of aluminum with a nickel-phosphorus chemical coating is of great importance in technology. Aluminum itself is difficult to coat and there is always a risk of the coating peeling off, even over time. Corrosion under the coating can speed up this process significantly. The corrosion mechanism of coated aluminum is complex and multifaceted; to understand it, it is necessary to use electrochemical studies, electron microscopy and X-ray phase analysis. Electrochemical tests were carried out under simulated cathode and anodic conditions simulating PEMFC (polymer electrolyte membrane fuel cells) fuel cell systems.
Nichrome - the power of nickel
Nichrome is also included in the group of precision alloys. Mainly composed of nickel (Ni) and also chromium (Cr).
The approximate percentage of each element in this alloy: nickel (Ni) 55-80%, chromium (Cr) 15-23%, manganese (Mn) no more than 2%. Also, a very small percentage is occupied by silicon (Si), titanium (Ti), aluminum (Al) and carbon (C), and even smaller amounts contain phosphorus (P) and sulfur (S).
Nichrome also has several grades, depending on changes in composition. Thus, nichrome brands such as X20N80 or X15N60 are widely used.
“X” means chromium, the numbers immediately after this letter are the percentage of chromium, respectively. So exactly “N” is the percentage of nickel. There are also brands with zirconium (Zr) additives - nichrome X20N60-N and X15N60-N.
By the way, the name of the brand can often hide the method of creating the alloy. For example, the names Nichrome X20N80-VI and Nichrome X15N60-VI imply that VI is a vacuum-induction production of the alloy.
Use of nickel in pure form
To protect metals from corrosion
For this purpose, coatings are used that are applied by electroplating or cladding. The first method is used for aluminum, cast iron, magnesium and zinc, the second - for unalloyed steels and iron.
For the production of metal products that have permanent shapes and high corrosion resistance
Nickel in its pure form is more expensive than iron and steel, so it is used in cases where it is impossible to get by with another metal with a nickel coating. Nickel is used to make crucibles and boilers, tanks for transporting and melting alkalis, storing reagents, food products, etc. Condensates are made in nickel pipes. Tools made from this metal are resistant to interaction with aggressive elements, so they are practically indispensable in chemical laboratories and medical centers. Various nickel devices are used for television, radar and nuclear technology.
As catalysts and filters in the chemical industry
Nickel has the same catalytic properties as palladium, but costs significantly less, so it is widely used in powder form in the hydrogenation reactions of alcohols, unsaturated and aromatic hydrocarbons, and cyclic aldehydes.
Pure nickel powder is also suitable for creating porous filters, which are used to filter various products: fuels, gases, etc.
For mechanical neutron beam choppers.
The properties of nickel make it possible to obtain high-energy neutron pulses, as a result of which plates made of this metal are used in nuclear physics.
Nickel is also used in the manufacture of electrodes in alkaline batteries.
Fechral and nichrome: physical properties
The presented alloys have specific physical properties. The density of fechral is 7.2 g/cm3, nichrome - 8.4 g/cm3.
The resistivity is 1.2-1.3 μOhm m and 1.0-1.1 μOhm m, respectively.
Fechral is ferromagnetic, while nichrome is non-magnetic.
The specific heat capacity of fechral is 0.48 °C, and that of nichrome is 0.44 - 0.46 °C. Hardness is 200-250 units and 140-150 units, respectively.
The maximum melting point of fechral is 1500 °C, nichrome is 1400 °C.
Main characteristics of aluminum alloys
Aluminum is a well-known element with a low density (2.7 g/cm³) and a low melting point (about 660 °C). Such a property as high ductility greatly facilitates rolling, forging, drawing and other processing of this metal. In addition, it is characterized by good electrical and thermal conductivity.
Cast aluminum alloys are cast in induction furnaces using special technologies. The starting material can be not only primary, but also secondary raw materials, for example, shavings. In the latter case, the scrap undergoes thorough preliminary preparation in order to clean it from dirt, oil and other foreign matter.
Cast and wrought aluminum alloys have the following properties:
- corrosion resistance;
- low linear shrinkage;
- ability to maximally fill the mold capacity;
- high resistance to cracks, cavities, gas porous voids and other defects;
- good fluidity, allowing you to create workpieces with complex configurations.
For industrial needs, aluminum alloys are supplied in ingots and castings. The specific properties of the material depend on the type of additives used and their percentage in the bulk.
Oxidation resistance
Due to the nickel composition, nichrome practically does not undergo oxidation. During the heating period, a thin but protective film (of chromium oxide) is formed on the surface of the nichrome part, which quite significantly increases the durability of the alloy in more severe conditions.
In turn, fechral will be subject to oxidation much more strongly, since the main component of the alloy is iron. During oxidation, a more massive, high-density oxide film is formed on the surface of the fechral alloy. This film appears much faster on fechral than on nichrome.
Hardness and ductility under normal conditions
Higher ductility is characteristic of nichrome. This percentage indicator for the alloy is approximately 20, but this may also depend on the marking.
Fechral has a ductility that is approximately 16%, but this is for the X15Yu5 brand. But the alloy marked X23Yu5T has even lower ductility - 10%.
But fechral is more durable because it contains a large amount of chromium. This does not allow it to be as flexible as nichrome.
Fechral is more brittle because it contains a high percentage of chromium.
Winding of fechral is available only at high temperatures (from 300 °C), and nichrome can be wound into a spiral at ordinary room temperature.
A little history
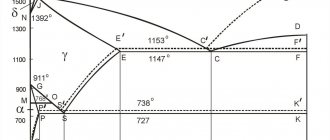
Invar is an alloy of iron and nickel, which contains 36% alloying additive. It was first discovered in France in 1896 by physicist Charles Guillaume. At this time, he was working on the search for inexpensive metal for standards of mass and length measures, which were made from a very expensive platinum-iridium alloy. Thanks to this discovery, the scientist received the Nobel Prize in physics in 1920.
The word "invar" in Latin means unchanging. This means that the coefficient of thermal expansion of an iron-nickel alloy remains constant over a wide temperature range - from -80 to 100 degrees Celsius. This alloy has several other names: nilvar, vakodyl, nilo-alloy, radiometal. Invar is a trademark of Imphy Alloys Inc., which is owned by the steel company Arcelor Mittal.
Scope of application of fechral and nichrome
Nichrome is considered a very durable alloy. This played a big role in the manufacture of different diameters of spirals and wires. Rods, nichrome threads, tapes, sheets, and strips are also made from nichrome.
Due to their high heat resistance, it is permissible to use nichrome alloys in the production of heating devices.
Nichrome retains its key physical properties even with strong temperature fluctuations, which makes it possible to use the alloy quite widely.
Tubular heating elements are made from nichrome and fechral. These alloys can be worked at a maximum temperature of 1400 °C and 1500 °C, respectively. Therefore, these alloys can be used in the production of rheostat elements and wire resistors.
Fechral and nichrome are used in similar areas, despite their different composition and distinctive characteristics.
About the properties of iron
Pure iron is silver-gray in color and has ductility and malleability. Native ingots found in nature have a pronounced metallic luster and significant hardness. The electrical conductivity of the material is high; it easily transmits current with the help of free electrons. The metal has average refractoriness, softens at a temperature of +1539 degrees Celsius and loses its ferromagnetic properties. This is a chemically active element. At normal temperatures it easily reacts, and when heated, these properties increase. In air it becomes covered with a film of oxide, which interferes with the continuation of the reaction. When exposed to a humid environment, rust appears, which no longer prevents corrosion. But despite this, iron and its alloys are widely used.