molybdenum powder mp47
Molybdenum alloys
NIMO 28
Molybdenum alloys
NIMO 20Avh
Molybdenum alloys
Tungsten-Molybdenum alloys
Molybdenum alloys
Any molybdenum alloys are classified as heavy, given the presence of a refractory metal as a base. Pure molybdenum with additives or a compound alloyed with other metals has high strength characteristics and is resistant to external environmental factors, corrosion, and extreme high temperatures.
Chemical properties and characteristics
Molybdenum occupies a special place among metals. With its help, it is possible to obtain alloys that are used in precision measuring instruments, counterweights, jet engines, melting furnace screens, in a wide variety of mechanisms and critical installations.
Mo is located in the 5th group and 5th period in the periodic table of chemical elements. The density at normal room temperature is 10,200 kg/m3, and the melting point reaches 2620±10°C. It imparts amazing properties to alloys: heat resistance, strength, reliability, low coefficient. expansion when exposed to high temperatures, insignificant neuronal capture cross section. At the same time, in terms of thermal conductivity, it is inferior to copper, but ahead of iron. In terms of processing, it is simpler compared to tungsten. But the latter refractory metal exhibits better mechanical strength.
In terms of their properties and characteristics, molybdenum alloys are as close as possible to pure metal, especially if the base occupies a large percentage of the total mass. Tungsten-molybdenum alloys are endowed with the best properties of both elements. By varying the ratios of refractory metals in one compound, it is possible to obtain a semi-finished product or a finished product with the required parameters.
Technologists point out that one of the significant disadvantages of Mo is its susceptibility to oxidation at temperatures above 500°C. At the same time, although alloying does not completely solve this problem, it helps to increase heat resistance and reduce fragility (for example, by introducing lanthanum oxide), and increase the time the part is exposed to increased load. When certain components are added, the recrystallization time increases.
Types and features of alloys
This category of the production catalog presents various compounds in the form of powder and finished rolled products. Let's reveal some of them:
• Tungsten-molybdenum. From compounds based on refractory metals, crucibles and extruded blanks, hot-rolled sheets, plates, rings, parts for equipping high-temperature and hydrogen furnaces, and sputtering targets are obtained. With certain processing, it is possible to obtain products of complex shapes. • Nickel-molybdenum alloys. The most common combination, available in various brands. Applicable for alloying steels, they are common in the manufacture of containers/vessels for radioactive elements, having a greater absorption coefficient of gamma rays than lead. Alloying in this case is more economical when compared to using pure Mo. At the same time, the characteristics of the finished products are almost identical. Collimators, dosimetric equipment and protective blocks/screens are also made from such alloys. • Chromium-molybdenum compounds. Chromium increases the strength of the joint, making it heat-resistant and acid-resistant. Alloys with the addition of cobalt are used in the production of artificial teeth, crowns, and bridges. Solid, but at the same time moderately elastic compounds do not corrode and do not react with biological fluids, food and drinks.
In addition to purchasing molybdenum alloys with nickel, tungsten and other metals, it is possible to order additional services - processing semi-finished and finished parts by various mechanical and chemical methods to give them certain qualities.
Comparison of alloys Monel NMZhMts 28-2.5-1.5 and Monel 400*
Monel is a copper-nickel alloy created at the beginning of the 20th century. Its peculiarity is that Monel metal is smelted from sulfide copper-nickel ore without prior separation of copper and nickel. This alloy production technology can significantly reduce the cost of Monel metal.
Figure 1. Copper-nickel ore
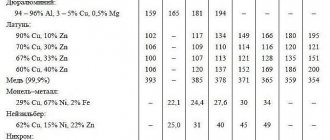
Monel is a structural corrosion-resistant alloy. The main components of this alloy are copper (Cu) - 27-29%, iron (Fe) - 2-3%, manganese (Mn) - 1.2-1.8%, nickel (Ni) + cobalt (Co) - the rest (according to GOST 492-2006).
Monel (NMZhMts 28-2.5-1.5) is characterized by high corrosion resistance and high mechanical properties.
The technological properties of this copper-nickel alloy, namely ductility, allow it to be processed by pressure in hot and cold states. Monel is used for the manufacture of wire, rods, strips, strips and sheets of various sizes. Monel pipe can also be made. The density of Monel metal is 8.8 g/cm3, the linear expansion coefficient is 14-15 10-6, the electrical resistivity of Monel is 0.425 Ohm mm2/m, and the temperature coefficient of electrical resistance is 0.0019. Physical properties of Monel alloy
| Property name | Meaning |
| Melting point, °C | 1350 |
| Density, g/cm3 | 8,8 |
| Linear expansion coefficient at 25-100 °C at 25-300 °C | 14·10-6 15·10-6 |
| Heat capacity at 200-400 °C, cal/g °C | 0,127 |
| Thermal emissivity | 43 |
| Thermal conductivity at 0-100 °C, cal/cm·sec·°С | 0,06 |
| Temperature coefficient of thermal conductivity | 0,006 |
| Electrical resistivity, Ohm mm2/m | 0,425 |
| Temperature coefficient of electrical resistance | 0,0019 |
Monel metal, as well as alloys of this type with additions of aluminum and silicon, are the most resistant of the group of copper-nickel alloys with respect to corrosion.
In a clean atmosphere containing sulfur compounds and moisture, Monel metal becomes covered with a thin film from green to brown. Its corrosion rate in the atmosphere of rural areas is 0.00013-0.00005 mm/year, in the sea 0.0002-0.0008 mm/year, in the atmosphere of industrial areas 0.003-0.0015 mm/year. In natural soft and hard waters, its corrosion rate is insignificant even at elevated temperatures and aeration (less than 0.003 mm/year). In natural seawater, Monel metal corrodes at a rate of 0.025-0.008 mm/year. Monel metal and alloys of this type are resistant in solutions of neutral, alkaline and slightly acidic salts, carbonic, hydrochloric, sulfuric, nitric and acetic acids. Monel metal is resistant to most organic acids and practically does not corrode in neutral and alkaline solutions of organic compounds. Also, this alloy is resistant to alkali solutions and an atmosphere of dry gases at room temperature.
The properties of the Monel alloy determine its use. Monel metal, which has high corrosion resistance in many environments, is used for the manufacture of parts operating in all kinds of aggressive environments. The low corrosion rate in natural sea and fresh water has determined the widespread use of Monel alloy in shipbuilding. Also, this corrosion-resistant copper-nickel alloy has found application in the oil, chemical and medical industries. Monel pipes, for example, are used to create pipelines.
Chemical composition, properties and applications of Monel 400 alloy (Nicorros®)
Monel 400, also called Nicorros®, is a structural corrosion-resistant copper-nickel alloy. The chemical composition of Monel 400 alloy (Nicorros®) is as follows: copper (Cu) - 28.0-34.0%, iron (Fe) - 1.0-2.5%, manganese (Mn) - max 2.0 %, nickel (Ni) - min 63%.
It is worth noting that MONEL alloy (MONEL® 400, 401, 404, R-405, K-500) is a trademark owned by Special Metals Corporation (USA). At the same time, some European manufacturers produce products from an alloy called Nicorros®. MONEL® 400 and NICORROS® alloys are identical, have the same chemical composition, properties, applications, but different names.
Features of Nicorros® Alloy (Monel 400)
:
- high corrosion resistance in most chemical environments;
- good mechanical properties at temperatures up to 550 °C;
- good weldability (non-consumable tungsten electrodes are usually used for welding) and workability under pressure.
Physical properties of Monel 400 alloy
| Property name | Meaning |
| Melting point, °C | 1320-1350 |
| Density, g/cm3 | 8,8 |
| Linear expansion coefficient at 20-100 °C, 10-6/K | 13,9 |
| Specific heat capacity at 200-400 °C, J/kg K | 465-490 |
| Thermal conductivity at 0-100 °C, W/m s K | 26-29,5 |
Monel 400 (Nicorros®) alloy has excellent resistance to neutral and alkaline salts and is therefore used in salt production plants.
Its great advantage is that Nicorros® (Monel 400) can be used in contact with fluorine, hydrofluoric acid, hydrogen fluoride. Also, this copper-nickel alloy can be used in contact with dilute mineral acids (sulfuric, hydrochloric) if they are in an airless space. In addition to acids, Monel 400 exhibits high resistance to caustic alkaline environments and sea water. Nicorros® alloy (Monel 400) is available in the form of sheet, strip, wire, round and square rod.
Application areas for copper-nickel alloy Monel 400 (Nicorros®):
- pipes carrying water and steam generators in power plants;
- salt heater and evaporator;
- valves and heat exchangers exposed to oxygen at high temperatures, pressures and oxygen concentrations;
- heater pipes for monoethanolamine.
Comparison of the chemical composition of the Monel NMZhMts 28-2.5-1.5 alloy and the Monel 400 alloy
| C | S | Ni+Co | Mn | Si | Ti | Cu | Fe | Al | Mg | As | Bi | Pb | P | Sb | |
| NMZHMts 28-2.5-1.5 | 0,2 | 0,01 | 65,2-69,8 | 1,2-1,8 | 0,05 | — | 27-29 | 2-3 | — | 0,1 | 0,01 | 0,002 | 0,003 | 0,01 | 0,002 |
| Monel 400 | 0,13 | 0,002-0,004 | 64,6-64,85 | 1,0 | 0,14 | 0,03 | 32,2 | 1,64-1,72 | 0,07-0,08 | — | — | — | — | — | — |
Comparison of the physical and mechanical properties of the Monel NMZhMts 28-2.5-1.5 alloy and the Monel 400 alloy
| Density, g/cm3 | Melting point, °C | Tensile strength, MPa | Relative extension, % | |
| NMZHMts 28-2.5-1.5 | 8,8 | 1350 | 590 | 35 |
| Monel 400 | 8,8 | 1350 | 551 | 45 |
Having analyzed the chemical composition, physical and mechanical properties, characteristic features and applications of alloys Monel 400 (Nicorros®) and Monel NMZhMts 28-2.5-1.5, we can conclude that these alloys are very similar. If necessary, Monel NMZhMts 28-2.5-1.5 alloy can be replaced with Monel 400 alloy.
How to buy molybdenum alloy profitably?
At ]LLC "New Technologies"[/anchor] you can order the production of heavy alloys based on refractory metals. You can buy molybdenum alloy of both common and rare brands. Before ordering, we recommend that you contact the company’s specialists. Many years of experience as a technologist and a well-functioning production line make it possible to strictly comply with GOST regulations in the production of powder, blanks in the form of ingots and bars, as well as any complex products made of chromium-molybdenum alloys, compounds containing nickel, tungsten, etc. metals in the composition. Call right now and find out about the possibility of placing an order for a batch of the required volume or for the production of parts according to individual drawings.
What properties does steel acquire as a result of alloying?
Each chemical element introduced into an alloy changes it. The proportions of impurities matter. In addition, one alloy is usually alloyed not with one metal additive, but with several.
Nickel alloying
In steel alloys, the metal nickel as an impurity helps ensure that austenite is formed and preserved in the alloy. This increases the strength of the alloy. If chromium and molybdenum are added to nickel, then nickel becomes even more effective for thermally strengthening steel, increasing its toughness, as well as fatigue strength. Ferritic steels are alloyed with nickel - they become more tough. Chromium-nickel austenitic steels are better resistant to corrosion.
Chromium alloying
Chromium is an element that, when added, improves the resistance of a metal alloy to the phenomena of oxidation and corrosion, makes the steel more durable even when heated to high temperatures, and also improves the ability of a high-carbon alloy to resist wear due to friction. In the process of alloying with chromium, chromium carbides are formed - thanks to them, the steel becomes harder and stronger: knives and other piercing and cutting tools can be made from it. If the steel also contains impurities of tin, arsenic, phosphorus or antimony, then they segregate to the boundaries of the “grains” of the alloy, which causes an increase in the temper brittleness of the steel alloy.
Alloying with molybdenum
Molybdenum creates greater thermal hardening during steel tempering (after hardening). Steels containing molybdenum at high temperatures are characterized by less creep.
Also, when molybdenum is included, the grain size of the alloy decreases and the steel becomes stronger. The indicator of resistance to corrosion processes (including pitting corrosion) improves.
By combining metal additives using alloying technology, chromium-nickel-molybdenum, chromium and chromium-nickel alloys are obtained, which have optimal sets of parameters for certain operating conditions and processing methods.
Refractory metal molybdenum
Molybdenum and its alloys are refractory materials. For the manufacture of shells for the warheads of missiles and aircraft, refractory metals and alloys based on them are used in two versions. In one embodiment, these metals serve only as heat shields, which are separated from the main structural material by thermal insulation. In the second case, refractory metals and their alloys serve as the main structural materials. Molybdenum ranks second after tungsten and its alloys in terms of strength properties. However, in terms of specific strength at temperatures below 1350-1450°C, Mo and its alloys take first place. Thus, molybdenum and niobium and their alloys, which have greater specific strength up to 1370°C compared to tantalum, tungsten and alloys based on them, are most widely used for the manufacture of skins and frame elements of missiles and supersonic aircraft. Mo is used to make honeycomb panels in spacecraft, heat exchangers, the shells of rockets and capsules returning to earth, heat shields, wing edge skins, and stabilizers in supersonic aircraft. Some parts of ramjet and turbojet engines (turbine blades, tail skirts, nozzle flaps, rocket engine nozzles, control surfaces in rockets with solid fuel) operate under very difficult conditions. At the same time, the material requires not only high resistance to oxidation and gas erosion, but also high long-term strength and impact resistance. At temperatures below 1370°C, molybdenum and its alloys are used to manufacture these parts.
Molybdenum is a promising material for equipment operating in sulfuric, hydrochloric and phosphoric acids. Due to the high resistance of this metal in molten glass, it is widely used in the glass industry, in particular for the manufacture of electrodes for glass melting. Currently, molybdenum alloys are used to make molds and cores for injection molding machines for aluminum, zinc and copper alloys. The high strength and hardness of such materials at elevated temperatures have led to their use as a tool in hot forming of steels and alloys (piercing mill mandrels, dies, press dies).
Molybdenum significantly improves the properties of steels. The Mo additive significantly increases their hardenability. Small additions of Mo (0.15-0.8%) to structural steels increase their strength, toughness and corrosion resistance so much that they are used in the manufacture of the most critical parts and products. To increase hardness, molybdenum is introduced into alloys of cobalt and chromium (stellites), which are used for surfacing the edges of parts made of ordinary steel subject to wear (abrasion). It is also part of a number of acid-resistant and heat-resistant alloys based on nickel, cobalt and chromium.
Another area of application is the production of heating elements for electric furnaces operating in a hydrogen atmosphere at temperatures up to 1600°C. Molybdenum is also widely used in the electronics industry and X-ray engineering for the manufacture of various parts of electron tubes, X-ray tubes and other vacuum devices.
Molybdenum compounds - sulfide, oxides, molybdates - are catalysts for chemical reactions, pigments for dyes, and components of glazes. Also, this metal as a microadditive is included in fertilizers. Molybdenum hexafluoride is used in the deposition of metallic Mo onto various materials. MoSi2 is used as a solid high-temperature lubricant. Pure single-crystal Mo is used to produce mirrors for high-power gas-dynamic lasers. Molybdenum telluride is a very good thermoelectric material for the production of thermoelectric generators (thermo-emf with 780 μV/K). Molybdenum trioxide (molybdenum anhydride) is widely used as a positive electrode in lithium power sources. MoS2 disulfide and molybdenum diselenide MoSe2 are used as a lubricant for rubbing parts operating at temperatures from -45 to +400°C. In the paint and varnish and light industries, a number of Mo chemical compounds are used as pigments for the production of paints and varnishes and for dyeing fabrics and furs.
Claim
1. Nickel-based nanocomposite for coating by heterophase spraying methods, containing chromium, molybdenum and silicon, characterized in that it additionally contains aluminum, zinc and titanium carbide in the following component ratio, wt.%:
| Chromium | 10,0-20,0 |
| Molybdenum | 25,0-45,0 |
| Silicon | 6,0-9,0 |
| Aluminum | 7,5-10,0 |
| Zinc | 1,5-2,0 |
| TiC | 2,0-4,0 |
| Nickel | rest, |
Moreover, the nanocomposite was obtained by introducing Al and Zn in the form of a master alloy at a component ratio of 5:1, respectively, and TiC in the form of nanoparticles with a size of 60-80 nm.
2. Nanocomposite according to claim 1, characterized in that it is a powder of the 50-80 micron fraction.
Nickel and its alloys
PROPERTIES AND APPLICATIONS OF NICKEL
Nickel (Ni) is a silver-white metal, quite hard and tough, which has wide application and importance in technology. It was discovered in 1751. The name of the element comes from the second part of the name of the mineral “kupfernickel” - false copper.
Nickel consists of a mixture of five isotopes with mass numbers 58, 60, 61, 62, 64. In addition, six artificial radioactive isotopes of nickel with mass numbers 56, 57, 59, 63, 66, 66 have been obtained. A number of radioactive isotopes of nickel find practical application . The crystal structure of nickel is face-centered. The physical and mechanical properties of nickel are characterized by the following data:
| Atomic mass | 58,71 |
| Density at 20°C, g/cm3 | 8,9 |
| Temperature, °C | |
| melting | 1455 |
| boiling | 3000 |
| Latent heat, cal/g: | |
| melting | 73 |
| evaporation | 1450 |
| Linear expansion coefficient at 20–100°С, 1/deg | 0.00001s |
| Thermal conductivity at 0—100°С, cal/(cm·sec·deg) | 0,142 |
| Electrical resistivity, ohm mm2/m | 0,068 |
| Modulus of normal elasticity, kg/mm2 | 20000 |
| Shear modulus, kg/mm2 | 7300 |
| Elastic limit of annealed nickel, kg/mm2 | 8 |
| Nickel yield strength, kg/mm2: | |
| annealed | 12 |
| deformed | 70 |
| Tensile strength of nickel, kg/mm2: | |
| annealed | 40—50 |
| deformed | 70-90 |
| Elongation of nickel, %: | |
| annealed | 35—40 |
| deformed . · · . | 2-4 |
| Hardness of NV nickel, kg/mm2: | |
| cast | 60—70 |
| annealed | 70—90 |
| deformed | 200 |
| Impact strength of annealed nickel. kg/mm2. | 18 |
| Nickel fatigue limit based on 107 cycles. kg/mm2: | |
| annealed | 16,6 |
| deformed | 29 |
Nickel has valuable chemical and mechanical properties. Good plasticity makes it possible to obtain various products from it by deformation in cold and hot states.
Nickel is one of the most active catalysts among metals.
Additions of nickel to other metals significantly change their properties and create opportunities for obtaining a wide range of various very valuable materials. Therefore, the main area of application of nickel is various alloys. More than 3,000 alloys are known that contain nickel. The production of nickel alloys is based on various types of interactions in which nickel enters with other elements.
Continuous solid solutions with nickel produce manganese, iron, cobalt, copper, palladium, rhodium, iridium, and platinum. Limited solid solutions with nickel form beryllium, boron, carbon, magnesium, aluminum, silicon, phosphorus, titanium, vanadium, chromium, zinc, gallium, germanium, arsenic, zirconium, niobium, molybdenum, ruthenium, indium, tin, antimony, lanthanum, tantalum, tungsten, rhenium, osmium, bismuth and uranium.
Various compounds form hydrogen, nitrogen, oxygen, sulfur, selenium, tellurium, fluorine, chlorine, bromine and iodine with nickel. Helium, neon, argon, krypton, xenon, radon, lithium, sodium, potassium, rubidium, cesium, francium, calcium, strontium, barium and iridium do not interact with nickel.
Nickel in its pure form is used as anti-corrosion protective coatings applied by cladding and electroplating. Nickel cladding is used to protect iron and unalloyed steels from corrosion by producing two- and three-layer metal. This significantly reduces the cost of products made from this metal instead of products made from pure nickel. Electrolytic nickel coatings are applied to aluminum, magnesium, zinc and cast iron.
Various apparatus, instruments, boilers and crucibles with high corrosion resistance and constancy of physical properties are also made from pure nickel, and reservoirs and tanks are made from nickel materials for storing food products, chemical reagents, essential oils, for transporting alkalis and other chemicals and food products, for melting caustic alkalis.
Nickel pipes are used for the manufacture of capacitors in hydrogen production and for pumping alkalis in chemical production. Chemically resistant nickel instruments are widely used in medicine and scientific research. Nickel is used for radar, television, and remote control of processes in nuclear technology. Nickel plates are used in mechanical neutron beam interrupters to produce high-energy neutron pulses.
Powdered nickel is used in catalytic processes, in hydrogenation reactions of unsaturated hydrocarbons, cyclic aldehydes, alcohols, and aromatic hydrocarbons. The catalytic properties of nickel are similar to those of platinum and palladium. Therefore, nickel, as a cheaper material, is widely used instead of these metals as a catalyst in hydrogenation processes.
Porous filters are made from pure nickel powders for filtering gases, fuels and other products in the chemical industry. Powdered nickel is also used in the production of nickel alloys and as a binder in the manufacture of hard and superhard materials.
Nickel is used as battery electrodes in alkaline batteries.
Nickel is used in alloys mainly in combination with iron and cobalt. It is an alloying element in various structural steels, as well as in magnetic and non-magnetic alloys, alloys with special physical properties, stainless and heat-resistant steels. Nickel-based alloys in combination with chromium, molybdenum, aluminum, titanium, and beryllium are widely common.
A large group of alloys are nickel-based alloys on a copper base - such as monel, nickel silver, brass and bronze. Nickel is widely used in cast iron.
Monel copper-nickel alloy, containing 68-70% Ni and 28-30% Cu, has very high corrosion resistance in acids and alkalis, in humid and marine atmospheres and is therefore used in the chemical and electrical industries, in marine equipment, in the production and storage of food products and in medicine. It is also used for (cladding of iron and steel.
Nickel and nickel-based alloys play an important role in the designs of some types of high-power nuclear reactors. Nickel alloys are used in nuclear reactors as protective high-temperature shells to protect uranium rods from corrosion.
Of great importance are Invar-type alloys with a low expansion coefficient, as well as Invar-type alloys with the addition of cobalt (Kovar). Nickel cast heat-resistant alloys are used in the construction of stationary gas turbines and aircraft jet engines.
NICKEL ALLOYS
Nickel-based alloys are used for electrical purposes and also as acid-resistant, heat-resistant and heat-resistant materials.
For electrical purposes, manganese nickel wire of the NMTs2.5 and NMTs5 grades is used for spark plugs of automobile, aircraft and tractor engines; made of alumel and chromel T alloys for thermocouples; made of chromel K alloy for compensation wires.
Acid-resistant nickel alloys. Materials in this group are nickel-based alloys alloyed with chromium, tungsten, molybdenum, copper and other elements. Nickel alloys alloyed with chromium and tungsten are resistant in aggressive oxidizing environments, and alloys that do not contain chromium (nickel-copper and nickel-molybdenum) are resistant in aggressive non-oxidizing environments. To increase corrosion resistance, nickel alloys are alloyed with silicon, aluminum and other elements.
Monel alloy. This alloy belongs to the acid-resistant nickel-based alloys containing copper as the main alloying element. It has very high corrosion resistance, high tensile strength and good ductility in cold and hot conditions. Monel metal is practically not subject to corrosion in dry air and distilled water, and is resistant to the action of dilute sulfuric acid, strong alkalis, most organic acids, dry gases at ordinary temperatures and sea water. Its chemical composition according to GOST 49 (2-52) is indicated in Tables 350 and 351. Monel metal is widely used for the manufacture of products that require high corrosion resistance and mechanical strength - in the chemical, shipbuilding, medical, oil, textile and other industries branches of machine and apparatus manufacturing.
Sheets, strips, tapes, rods, wire, and pipes are made from Monel metal grade NMZhMts 28-2.5-11.5.
Monel K is a regular Monel metal alloyed with aluminum and strengthened by heat treatment. It is used in cases where higher strength than conventional Monel metal is required: for pump valves,
springs and other parts of high strength and high corrosion resistance. It is not recommended to use this alloy for operation at temperatures above 315°C in environments containing sulfur compounds. Forgings, rods, strips, and pipes are made from the alloy.
Compared to regular Monel metal, Monel S contains an increased amount of silicon (3-5%). It is used for casting parts that require high strength, hydraulic density, high chemical resistance and good abrasion resistance: valve seats, rubbing parts of gas turbines and other machines.
Inconel is a nickel alloy containing chromium and iron as the main alloying elements, used for parts operating in oxidizing environments and at high temperatures. It is not recommended to use this alloy for parts operating at temperatures above 815°C in environments containing sulfur compounds.
A large group of acid-resistant nickel alloys are alloys that contain molybdenum as one of the main alloying elements.
Hasteloy A (EI460). The main alloying elements in the alloy are molybdenum and iron. It is used for equipment parts operating in hydrochloric acid at temperatures up to 70°C, in diluted (up to 50%) sulfuric acid up to boiling. The best combination of corrosion resistance and toughness of the alloy is achieved after quenching from 1150-1175°C in water or air. It is not recommended to use the alloy in oxidizing environments.
Hastelloy V (EI461). This alloy has a higher molybdenum content than Hastela A. In addition, it contains vanadium. The alloy is used for the manufacture of parts operating in hydrochloric acid of all concentrations, heated up to the boiling point, as well as in other non-oxidizing acids and in air at temperatures up to 760°C. The optimal combination of properties of the alloy is achieved after quenching in water or air.
Hasteloy S (EP375) is a nickel-based alloy in which the main alloying components are molybdenum, chromium, tungsten and iron. This alloy is intended for the manufacture of equipment parts operating at medium temperatures in the following oxidizing environments: wet chlorine, hypochlorites, ferric chloride and copper chloride, nitric and phosphoric acids, mixtures of hydrochloric acid with sulfuric acid under oxidizing conditions, sea water, acetic and formic acids and their salts. When working in air, the alloy can be used up to 1090 Celsius. The alloy is not recommended for use in nitric acid at temperatures above LC.
Hastelloy D is a nickel-based alloy; it contains silicon and copper as the main alloying elements. It is used to produce parts by casting into the ground or into a chill mold, working with hot solutions of sulfuric acid of all concentrations at temperatures up to 70°C. It is not recommended to use this alloy for work in oxidizing environments. Due to its high hardness, the alloy is difficult to process by cutting. To improve machinability, the alloy is annealed at temperatures of 1050-1080 °C and then slowly cooled
Hasteloy F is an alloy whose main components are nickel, iron, chromium, molybdenum, thallium and niobium. It is used to make parts that work in contact with acids and alkalis under redox conditions. It has good resistance to stress corrosion in chloride solutions. Semi-finished products from this alloy are supplied in the form of sheets, rods and castings.
Nionel is a nickel alloy whose main components are nickel, molybdenum, chromium, iron, copper and titanium. The alloy is used for the manufacture of containers for storing phosphoric and sulfuric acids, as well as hot solutions of caustic soda.
Illium G is an alloy of nickel and chromium, alloyed with aluminum, molybdenum, iron, tungsten, and copper. The alloy has good resistance to sulfuric, phosphoric, nitric and organic acids, mixtures of mineral acids and salts, as well as sea water, fluoride and sulfur compounds. The alloy is used in chemical engineering for high-strength cast parts - for pumps and for viscose production equipment. It is not recommended to use the alloy for parts working in contact with halogens and their acids.
Heat-resistant nickel alloys
Alloys of nickel and chromium with the addition of other alloying elements - titanium, aluminum, molybdenum, tungsten, niobium, strontium, etc. - are widely used as heat-resistant deformable materials. These alloys are used to manufacture the most stressed parts of gas turbine engines and other power plants. The properties of nickel heat-resistant alloys are highly dependent on the heat treatment regime.
Nickel cast heat-resistant alloys have higher long-term strength limits than similar alloys in the deformed state. This is due to the peculiarities of crystallization of alloys, accompanied by the formation of carbide and boride phases along grain boundaries, which impede the development of cracks along these boundaries. Casting alloys are subject to alloying to a greater extent than wrought alloys, since in the latter it is limited by the need to apply hot plastic deformation, which is very difficult with heavy alloying. Casting alloys also have greater manufacturability than wrought alloys, especially in the manufacture of products of complex shapes. However, cast alloys have lower toughness than wrought alloys. By improving the quality of the ingot and using advanced methods of hot machining, the gap between the possible temperature level of performance of heat-resistant alloys in the cast and deformed states has been significantly reduced.
Alloys of the KhN717TYU and KhN77TYUR grades are used for the manufacture of working blades and disks of gas turbines. In an aged state, these alloys have higher strength and hardness, but lower ductility and impact strength. They have high heat resistance characteristics up to 750°C. At higher temperatures, their reliable operation is maintained at reduced loads.
Alloys KhN77TYu and KhN77TYUR have high resistance to fatigue and oxidation and low notch resistance. When heated for a long time before quenching, the surface layers of the alloys become depleted of chromium, titanium and aluminum, so when making gas turbine blades from them, the depleted layer must be removed. To ensure high heat-resistant and performance properties, it is necessary to achieve uniform metal grains with a diameter of 0.5-1.0 mm by forging and stamping.
Blades of gas turbine engines operating at 800-850°C are made from alloys of grades KhN70VMTYu and KhN70MVTYUB. After machining, the parts are subjected to heat treatment. (The parts are heated in an argon atmosphere, and additional aging is carried out in a normal air environment. After this treatment, the parts become insensitive to notching.
Alloy KhN67MVTYu is intended for working blades of gas turbines operating at temperatures of 770-850°C.
Heat-resistant wrought nickel alloys are superior to nickel in their resistance to oxidation at high temperatures. They have high technological ductility and good weldability. The heat resistance of nickel is usually increased by adding chromium. Nickel alloys with chromium (nichrome) contain from 15 to 30% Cr. In addition, aluminum and other alloying elements are introduced into the composition of nickel heat-resistant alloys to increase heat resistance.
Nickel wrought heat-resistant alloys are used for the manufacture of parts operating at temperatures of 700–1100°C.
Nichromes are used as structural heat-resistant materials, which, along with high heat resistance, have increased heat resistance. The heat resistance of these alloys is increased by alloying with refractory elements that form stable carbides or carbonitrides (niobium, titanium).
Wrought heat-resistant nickel alloys, which have a satisfactory ability to cold deformation, are suitable for the manufacture of sheet parts by deep drawing and bending.
Application of alloys:
ХН78Т - flame tubes of combustion chambers of gas turbines operating at 700-900°C;
KhN75MBTYU - flame tubes of combustion chambers of gas turbines, afterburners of jet engines operating at 700-900°C;
ΧΗ60В - flame tubes of combustion chambers of turbines, afterburners, afterburner valves of engines operating at 850-1000°C;
KhN70Yu and KhN60Yu are pockets for flame tube mixers that require heat resistance up to 1100°C.
Last year's jump in nickel prices by more than 200% has reignited talk of a possible replacement, particularly with molybdenum, which is also used to make corrosion-resistant steels. And although not all analysts agree with the reality of the threat to the nickel market from alternative metals, this factor certainly influences the growth of molybdenum consumption. In this regard, the prospects for this metal are becoming increasingly important for investors. According to InfoMine.com, the price of molybdenum is currently around $30/lb - about six times higher than four years ago. This is the highest price since November 2005, although the all-time high was reached in June 2005, when prices exceeded $45/lb.
The explanation for high prices is the supply factor. In 2005, global molybdenum production was 416 million pounds (188.7 thousand tons) and demand was 400 million pounds (181.4 thousand tons), according to the International Molybdenum Association's January newsletter. A significant portion of molybdenum is obtained as a by-product of copper production. As a result, a decrease in copper output in the early 2000s. caused a reduction in the amount of available molybdenum. As Martin Hayes, an analyst at BaseMetals.com, notes, even now, almost all copper producers view molybdenum only as a byproduct and do not make additional efforts to increase its production.
James Finch, editor-in-chief of StockInterview.com, noted that nickel is being replaced in tiny quantities to cut additional steel production costs, which have risen more than 70%. Therefore, reducing the nickel content and adding other metals such as chromium, manganese and molybdenum reduces the impact of nickel on the final cost of production.
In stainless steels, molybdenum is used along with chromium. According to the International Molybdenum Association, about 10% of the world's stainless steel contains molybdenum.
Although many analysts agree that the steel industry is using molybdenum as a partial substitute for nickel in certain grades of stainless steels, the extent of this phenomenon is somewhat unclear. Lawrence Roulston, editor of Resource Opportunities, notes that the molybdenum market is much smaller than the nickel market, and there are fewer new potential sources of supply. Thus, it is difficult to imagine that molybdenum could become a significant replacement for nickel. In addition, replacing one metal with another is a long-term process.
Molybdenum is more abundant than nickel, but it also has short-term supply issues, according to Eric Coffin, co-editor of HardRockAnalyst.com. Until a couple of new large plants come online in a year or two, molybdenum will do nothing to help nickel consumers.
According to Ian McDonald, executive chairman of Blue Pearl Mining Ltd., global demand for molybdenum has grown at a rate of more than 4% per year over the past 50 years, and last year it increased by 6%, while in China it jumped by 20%. In 1993, the molybdenum market was 230 million pounds, with China producing 110 million pounds of molybdenum and exporting 100 million pounds. Now China produces 80 million pounds (about 36 thousand tons), exporting 30-40 million pounds (13.5-18 thousand tons).
As MacDonald noted, the areas of molybdenum consumption are expanding. So, 25 years ago it was not used in cars. Now there are 15 small parts that use molybdenum, and 50 million cars were produced last year. The potential increase in demand for molybdenum comes from its use in nuclear plants. Depending on the design, each installation requires 500-800 thousand pounds (227-363 tons) of molybdenum, and the nuclear reactor itself consists of approximately 6% of it. So far, the only major use of molybdenum is pipelines.
As global molybdenum production continues to struggle to expand, global demand for the metal is booming, analysts say. According to Kevin Bambrough, market analyst at Sprott Asset Management Inc., there is a good chance the molybdenum price will reach its recent high of $40/lb. However, it would not be a surprise if it exceeds this maximum.