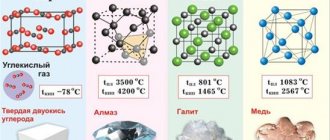
Metals melt, as a rule, at a very high temperature, which can reach more than 3 thousand degrees. Although some of them can be melted at home, such as lead or tin. But mercury is melted at a temperature of minus 39 degrees. This cannot be achieved at home. Melting point is one of the important indicators of the production of not only the metal itself, but also its alloys. When smelting raw materials, specialists take into account other physical and chemical properties of the ore and metal.
Iron and its properties
Iron is a chemical element that is number 26 on the periodic table. It is one of the most abundant elements in the entire solar system. According to research materials, the Earth's core contains approximately 79−85% of this substance . There is also a large amount of it in the earth's crust, but it is inferior to aluminum.
In its pure form, the metal is white with a slightly silvery tint. It is plastic, but the impurities present in it can determine its physical properties. Reacts to a magnet.
Iron is present in water. In river waters its concentration is approximately 2 mg/l of metal. In sea water its content can be a hundred or even a thousand times lower.
Iron oxide is the main form that is mined and found in nature. Iron oxide can be located in the uppermost part of the earth's crust and be a component of sedimentary formations.
An element in twenty-sixth place on the periodic table can have several oxidation states. It is they who determine its geochemical feature of being in a certain environment. In the Earth's core, the metal is present in a neutral form.
Is metal melting physical or chemical?
Is the melting of graphite and metal a physical or chemical phenomenon? Why?
Answers and explanations 1
Physical phenomena, because melting under fire causes deformation. These are physical phenomena.
Do you know the answer? Share it!
How to write a good answer?
To add a good answer you need:
- Answer reliably those questions to which you know the correct answer;
- Write in detail so that the answer is comprehensive and does not prompt additional questions;
- Write without grammatical, spelling and punctuation errors.
- Copy answers from third-party resources. Unique and personal explanations are well appreciated;
- Answer not to the point: “Think for yourself,” “Easy,” “I don’t know,” and so on;
- Using swear words is disrespectful to users;
- Write in UPPER CASE.
Mining
There are several ores containing iron. However, the following are mainly used as raw materials for iron production in industry:
- magnesite ore;
- goethite ore;
- hematite ore.
And also the following types of ore are often found:
- lellingitis;
- siderite;
- marcasite;
- ilmenite;
- is violent.
There is also a mineral called melanterite . It is used primarily in the pharmaceutical industry. It consists of green, fragile crystals with a glassy sheen. Medicines containing ferum are produced from it.
The main deposit of this metal is South America, namely Brazil.
Melting point table
It is important for anyone involved in the metallurgical industry, whether a welder, foundry worker, smelter or jeweler, to know the temperatures at which the materials they work with melt. The table below shows the melting points of the most common substances.
Read also: Self-tapping screw for concrete without drilling
Table of melting temperatures of metals and alloys
| Name | T pl, °C |
| Aluminum | 660,4 |
| Copper | 1084,5 |
| Tin | 231,9 |
| Zinc | 419,5 |
| Tungsten | 3420 |
| Nickel | 1455 |
| Silver | 960 |
| Gold | 1064,4 |
| Platinum | 1768 |
| Titanium | 1668 |
| Duralumin | 650 |
| Carbon steel | 1100−1500 |
| Cast iron | 1110−1400 |
| Iron | 1539 |
| Mercury | -38,9 |
| Cupronickel | 1170 |
| Zirconium | 3530 |
| Silicon | 1414 |
| Nichrome | 1400 |
| Bismuth | 271,4 |
| Germanium | 938,2 |
| Tin | 1300−1500 |
| Bronze | 930−1140 |
| Cobalt | 1494 |
| Potassium | 63 |
| Sodium | 93,8 |
| Brass | 1000 |
| Magnesium | 650 |
| Manganese | 1246 |
| Chromium | 2130 |
| Molybdenum | 2890 |
| Lead | 327,4 |
| Beryllium | 1287 |
| Will win | 3150 |
| Fechral | 1460 |
| Antimony | 630,6 |
| titanium carbide | 3150 |
| zirconium carbide | 3530 |
| Gallium | 29,76 |
In addition to the melting table, there are many other supporting materials. For example, the answer to the question what is the boiling point of iron lies in the table of boiling substances. In addition to boiling, metals have a number of other physical properties, such as strength.
Iron melting and required temperature
The melting point of a metal is the minimum temperature at which it changes from solid to liquid. At the same time, it remains practically unchanged in volume.
Metal can be produced from ore in various ways, but the most basic of them is blast furnace . In addition to blast furnace, iron smelting is also used by roasting crushed ore with an admixture of clay. From the resulting mixture, pellets are formed, which are processed in a furnace followed by reduction with hydrogen. Next, the iron is melted in an electric furnace.
The melting point of iron is very high. For a technically pure element it is +1539 °C. This substance contains an impurity - Sulfur, which can only be extracted in liquid form. Without impurities, pure material is obtained by electrolysis of metal salts.
Metal melting process
This process refers to the transition of a substance from a solid to a liquid state. When the melting point is reached, the metal can be in either a solid or liquid state; further increase will lead to the complete transition of the material into a liquid.
The same thing happens during solidification - when the melting point is reached, the substance will begin to transition from a liquid to a solid state, and the temperature will not change until complete crystallization.
It should be remembered that this rule applies only to pure metal. Alloys do not have a clear temperature boundary and undergo state transitions in a certain range:
- Solidus is the temperature line at which the most fusible component of the alloy begins to melt.
- Liquidus is the final melting point of all components, below which the first alloy crystals begin to appear.
It is impossible to accurately measure the melting point of such substances; the point of transition of states is indicated by a numerical interval.
Depending on the temperature at which metals begin to melt, they are usually divided into:
- Low-melting, up to 600 °C. These include tin, zinc, lead and others.
- Medium melting, up to 1600 °C. Most common alloys, and metals such as gold, silver, copper, iron, aluminum.
- Refractory, over 1600 °C. Titanium, molybdenum, tungsten, chromium.
There is also a boiling point - the point at which the molten metal begins to transition into a gaseous state. This is a very high temperature, typically 2 times the melting point.
Effect of pressure
The melting temperature and the equal solidification temperature depend on pressure, increasing with its increase. This is due to the fact that with increasing pressure the atoms come closer to each other, and in order to destroy the crystal lattice they need to be moved away. At increased pressure, greater thermal energy is required and the corresponding melting temperature increases.
There are exceptions when the temperature required to transform into a liquid state decreases with increased pressure. Such substances include ice, bismuth, germanium and antimony.
Classification of metals by melting point
Different metals can turn liquid at different temperatures. As a result, a certain classification is distinguished. They are divided as follows:
- Low-fusibility elements are those elements that can become liquid even at temperatures below 600 degrees. These include zinc, tin, lead, etc. They can be melted even at home - you just need to heat them up using a stove or soldering iron. Such types have found application in technology and electronics. They are used to connect metal elements and move electric current. Tin melts at 232 degrees, and zinc at 419 degrees.
- Medium-melting - elements that begin to melt at temperatures from six hundred to one thousand six hundred degrees. These elements are used mainly for building elements and metal structures, that is, when creating fittings, slabs and building blocks. This group includes: iron, copper, aluminum. The melting point of aluminum is relatively low and is 660 degrees. But iron begins to turn into a liquid state only at a temperature of 1539 degrees. It is one of the most common metals used in industry, especially in the automotive industry. However, iron is susceptible to corrosion, that is, rust, so it requires special surface treatment. It must be coated with paint or drying oil, and moisture must not be allowed to enter.
- Refractory are materials that melt and become liquid at temperatures above 1600 degrees. This group includes tungsten, titanium, platinum, chromium, etc. They are used in the nuclear industry and for some machine parts. They can be used for melting other metals, making high-voltage wires or wire. Platinum can be melted at 1769 degrees, and tungsten at 3420 °C.
The only element that is in a liquid state under normal conditions is mercury. Its melting point is minus 39 degrees and its vapors are poisonous, so it is used only in laboratories and closed containers.
Read also
Wood structure
Wood structure By making only a cross section, you can clearly see the structure of the wood. Each block of unhewn wood has bark - this is the skin of the tree, which is not used in work; it must be removed. Beneath the bark is the tree's growth zone, which
Wood structure
Wood structure By making only a cross section, you can clearly see the structure of the wood. Each block of unhewn wood has bark - this is the skin of the tree, which is not used in work; it must be removed. Beneath the bark is the tree's growth zone, which
LECTURE No. 1. Structure of wood
LECTURE No. 1. Structure of wood 1. Types of tree species and parts of wood Growing trees have the following components: roots, trunk, branches, leaves. The root system of trees acts as a supplier of moisture and nutrients from the soil along the trunk and branches to the leaves.
Macroscopic structure of wood
2. Macroscopic structure of wood With a cross section of a tree trunk, the main macroscopic features can be established: sapwood, core, annual layers, pith rays, vessels, resin ducts and pith repetitions. Young trees of all species have wood
Structure of metals
1. Structure of metals Metals and their alloys are the main materials in mechanical engineering. They have many valuable properties, mainly due to their internal structure. A soft and ductile metal or alloy can be made hard and brittle, and vice versa. In order to
Properties of glass melts
Properties of glass melts The properties of glass melts include viscosity, the associated hardening rate, surface tension and crystallization, as well as heat capacity, thermal conductivity, and electrical conductivity. The significance of these properties in production
§ 3.3 The structure of atoms and Mendeleev’s periodic law
§ 3.3 The structure of atoms and Mendeleev’s periodic law The properties of simple bodies, as well as the forms and properties of compounds of elements, are periodically dependent (or, expressed algebraically, form a periodic function) on their atomic weights. DI. Mendeleev It is believed that
§ 3.6 Structure of nuclei
§ 3.6 Structure of nuclei The more nucleons must fit in the nucleus, the greater the surface area of the nucleus, where protons and neutrons are added... These features are best met by the shape of the nucleus in the form of two Cheops pyramids connected
§ 4.14 Structure of matter and chemical bonding
§ 4.14 The structure of matter and the chemical bond What finally appears to us as hardened and dense must undoubtedly consist of hooked principles, linked together like intertwined branches. In this category of things, taking first place in it, There will be diamonds
Chapter 32 The Structure of Space – Time
Chapter 32 Structure of Space - Time “Action is the curvature of the World” Pavel Dmitrievich Uspensky, 1911 We have already assumed analogies of the quantum structure of the microworld and the macroworld, under certain conditions. Next, the laws of the resonant structure of our
Strength of metals
In addition to the ability to transition from a solid to a liquid state, one of the important properties of a material is its strength - the ability of a solid body to resist destruction and irreversible changes in shape. The main indicator of strength is the resistance that occurs when a pre-annealed workpiece breaks. The concept of strength does not apply to mercury because it is in a liquid state. The designation of strength is accepted in MPa - Mega Pascals.
There are the following strength groups of metals:
- Fragile. Their resistance does not exceed 50MPa. These include tin, lead, soft-alkaline metals
- Durable, 50−500 MPa. Copper, aluminum, iron, titanium. Materials of this group are the basis of many structural alloys.
- High strength, over 500 MPa. For example, molybdenum and tungsten.
Metal strength table
| Metal | Resistance, MPa |
| Copper | 200−250 |
| Silver | 150 |
| Tin | 27 |
| Gold | 120 |
| Lead | 18 |
| Zinc | 120−140 |
| Magnesium | 120−200 |
| Iron | 200−300 |
| Aluminum | 120 |
| Titanium | 580 |
The most common alloys in everyday life
As can be seen from the table, the melting points of elements vary greatly even among materials commonly found in everyday life.
Thus, the minimum melting point of mercury is -38.9 °C, so at room temperature it is already in a liquid state. This explains why household thermometers have a lower mark of -39 degrees Celsius: below this indicator, mercury turns into a solid state.
The most common solders in household use contain a significant percentage of tin, which has a melting point of 231.9 °C, so most solders melt at the operating temperature of the soldering iron 250−400 °C.
In addition, there are low-melting solders with a lower melt limit, up to 30 °C, and are used when overheating of the materials being soldered is dangerous. For these purposes, there are solders with bismuth, and the melting of these materials lies in the range from 29.7 - 120 °C.
Melting of high-carbon materials, depending on alloying components, ranges from 1100 to 1500 °C.
The melting points of metals and their alloys are in a very wide temperature range, from very low temperatures (mercury) to several thousand degrees. Knowledge of these indicators, as well as other physical properties, is very important for people who work in the metallurgical field. For example, knowledge of the temperature at which gold and other metals melt will be useful to jewelers, foundries and smelters.
Metals melt, as a rule, at a very high temperature, which can reach more than 3 thousand degrees. Although some of them can be melted at home, such as lead or tin. But mercury is melted at a temperature of minus 39 degrees. This cannot be achieved at home. Melting point is one of the important indicators of the production of not only the metal itself, but also its alloys. When smelting raw materials, specialists take into account other physical and chemical properties of the ore and metal.
Read also: Carburizing steel at home