There are four main uses of phase diagrams:
- Development of new alloys based on application requirements.
- Production of these alloys.
- Development and control of appropriate heat treatment procedures that are designed to improve the chemical, physical and mechanical properties of alloys.
- Eliminates problems encountered with new alloys, ultimately improving predictability of product properties.
When designing an alloy, phase diagrams help prevent designing too many variants, reducing costs and processing time. They also help develop alternative alloys, or the same alloys but with alternative alloying elements.
Component composition
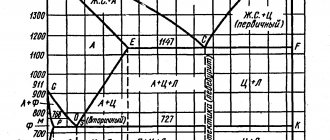
The iron-carbon diagram was developed to show the microstructural state of a metal as a function of temperature and carbon content. It shows that, depending on the intensity of steel cooling, very different microstructures can appear in it.
When the metal is molten, that is, in a liquid state, there are no lattice structures. Atoms can move freely in all directions. To achieve the desired crystal structures, it is necessary to allow the metal to cool slowly and completely at room temperature. Intermediate heat treatments have a positive effect on the formation of crystalline structures.
Along the vertical axis of the iron-carbon diagram is the temperature scale in °C. The metal is shown here from solid to liquid state. For example, pure iron begins to melt at 1147°C. The temperature of the stable state of the melt is 1536 °C.
The horizontal axis indicates the percentage of carbon in the metal.
The lines that connect the characteristic points of the diagram mark the transformation of the metal. They separate areas where the metal is in a state of transformation from solid to liquid. But at the same time, the iron-carbon phase diagram also determines the reorganization of atoms in the crystal lattice.
The diagram shows areas where the metal is only in the state. melt, ferrite or austenite. The remaining zones are characterized by a certain combination of properties. These are, for example, melt and austenite, austenite and ferrite, ferrite and pearlite.
The designation of intersection points in capital letters on the transformation lines is intended for better understanding. They indicate a transition to another state or condition.
The diagram assumes constant cooling of metals. If the metal is cooled slowly, large grains are formed, but if the metal cools quickly, the grain sizes decrease. The type of metal grain determines its strength properties. Coarse-grained material has lower strength, but it can be made finer and therefore stronger by heat treatment or plastic deformation (hardening). Fine-grained metal has the highest level of strength.
Let us briefly characterize the main chemical components of the diagram.
Iron
Iron forms a cubic spatial lattice. The iron atoms are located at the corners of this lattice. The amount of carbon that can be dissolved in iron depends on the modification of the iron because different crystal lattice shapes of iron (e.g., space-centered cubic and face-centered cubic) have interstitial spaces of different sizes.
A minor role is played by the delta-mixed crystal, which has body-centered atoms, but exists only in high-alloy steels. These crystals form in the temperature range from 1536 °C (the melting point of pure iron) to 1392 °C.
An alpha mixed crystal exists as a body-centered cubic lattice. These structures form in pure iron up to a temperature of 911 °C.
In a gamma mixed crystal, the iron atoms are face-centered, meaning that they are located in the middle of each face of the cube. These crystals form at temperatures ranging from 1392 °C to 911 °C in pure iron.
Carbon
In low concentrations, carbon does not form a solid chemical compound with iron, but is deposited in the interstices of the iron crystal lattice.
Carbon in iron is an interstitial impurity and can exist in the form of a face-centered or body-centered cubic lattice. The phase diagram of iron-carbon alloys establishes regions of stable existence of a solid solution with α, γ and δ iron phases.
The crystal lattices described above have different carbon solubilities depending on temperature. Carbon is deposited in the interstitial spaces of the crystal lattice, while the face-centered cubic lattice, also called austenite, has a solubility no more than one hundred times higher than ferrite crystals.
Cementite
Cementite is a metastable phase of an alloy with a fixed composition, having the chemical formula Fe3C. At room temperature, cementite very slowly decomposes into iron and carbon (graphite).
Several other factors (such as high temperatures and the addition of certain alloying elements) may influence this decomposition as they promote the formation of graphite.
Cementite is hard and brittle, which makes it suitable for hardening steel (there is a special technology for chemical-thermal treatment of steel called cementation). The mechanical properties of cementite depend on its microstructure, as well as on the conditions of bonding with ferrite.
The liquid Fe-C solution on the diagram is indicated above the L (liquidus) line. Since δ-ferrite melts at 1538 °C, it is obvious that the melting point of iron decreases with increasing carbon content.
Perlite
Perlite grains include structural associations where all the formed plates are parallel to each other. As the number of pearlite nodules increases, the growth in the size of the plates stops, and they themselves can change the direction of their movement. As the temperature decreases, perlite colonies turn into spherical, fine-grained formations.
Ledeburite in steels
Ledeburite is one of the main structural components of iron-carbon alloys and is a eutectic mixture of austenite and cementite that forms at 1145°C and below (for pure iron-carbon alloys). Austenite transforms into a ferrite-cementite mixture at temperatures below 723°C. In steels, ledeburite, consisting of austenite and carbides, is formed only at a very high content of alloy components and carbon (0.7...1.0%); Such steels (called ledeburite steels.
Metastable unalloyed ledeburite eutectic Fe – Fe3C is classified as quasi-regular. It has been proven that after the nucleation of Fe3C, the growth of cementite plates occurs rapidly, resulting in the formation of austenite at the joints, and an orientation relationship occurs. Fe3C and austenite also grow together and form a rod structure that is perpendicular to the cementite plates. These two growth modes form a quasi-regular structure, but growth at the edge is faster than at the sides and dominates the structure. Experiments on directional crystallization showed that the operating point on the growth curve of a quasi-regular structure is close to the extremum point. Such a quasi-regular structure can be modified by quenching, but modification of impurities has not yet been studied.
The structural substrate of ledeburite class steels has hypoeutectic components, including pearlite, ledeburite and nodular graphite. The doped layer consists of dendrites and interdendrites. In nodular cast iron, graphite nodules gradually dissolve in the melt pool, and at the same time, under the influence of hydrodynamic and fluid forces, move to the surface.
Carbon
Carbon
General characteristics of group IVa elements
From C to Pb (from top to bottom in the periodic table) there is an increase in: atomic radius, metallic, basic, reducing properties. Electronegativity, ionization energy, and electron affinity decrease.
Natural compounds
Receipt
Chemical properties
When heated, carbon reacts with many non-metals: hydrogen, oxygen, fluorine.
When heated, carbon reacts with metals, exhibiting its oxidizing properties. Let me remind you that metals can only take positive oxidation states.
Obviously, the degree of oxidation of carbon in combination with different metals may differ.
Carbon reduces not only metals from their oxides, but also nonmetals in a similar way:
It can also reduce its own oxide:
In reactions with acids, carbon acts as a reducing agent:
Dissolving in the blood, carbon monoxide (which has a 300 times greater affinity for hemoglobin than oxygen) easily outcompetes oxygen and takes its place in red blood cells. Carbon monoxide poisoning is often fatal.
In industry, carbon monoxide is produced by the reduction of carbon monoxide IV or gasification of coal (t = 1000 °C).
In the laboratory, carbon monoxide is obtained from the decomposition of formic acid in the presence of sulfuric acid:
Completely oxidizes to carbon dioxide in reaction with oxygen, reducing metal oxides.
Product of complete oxidation of carbon. Refers to acidic oxides, corresponds to carbonic acid H2CO3. Colorless gas, odorless.
In industry, carbon dioxide is obtained from the decomposition of limestone, during the production of alcohol, and from the alcoholic fermentation of glucose.
In laboratory conditions, the reaction of chalk (marble) with hydrochloric acid is used.
Carbon dioxide is formed when organic substances burn:
As a result of the reaction with water, unstable carbonic acid is formed, which immediately breaks down into water and carbon dioxide.
When heated, it is capable of oxidizing metals to their oxides.
Zn + CO2 → (t) ZnO + CO
Carbonic acid
A weak dibasic acid, existing only in solutions, decomposes into water and carbon dioxide.
This can be easily explained by remembering the ability of carbonic acid to form acid salts that are soluble.
Li2CO3 + CO2 + H2O → LiHCO3 (average salt + acid = acid salt)
To return the average salt, you should add alkali to the sour salt.
© Bellevich Yuri Sergeevich 2018-2021
Phases in the iron-carbon system
Phases are physically homogeneous states of an alloy. The phase has a precise chemical composition - a specific arrangement and connection between atoms. This atomic structure gives different properties to different phases.
Some special alloys can exist in several phases, which is achieved by heating the metal to certain temperatures and using different heat treatment procedures.
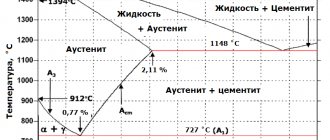
Liquid
Exists at temperatures that exceed 1457 oC. Phase transformation at this temperature means complete melting, so the liquid phase is always indicated by the line L on the diagram.
Ferrite
Present in the diagram in three different phases:
- The delta ferrite (δ-Fe) phase is a solid solution of C in δ-Fe (bcc) in the high temperature region of the diagram. The solution is stable above 1400 °C and melts above 1539 °C. Structurally similar to α-ferrite;
- The gamma ferrite (γ-Fe) phase is a solid solution of C in γ-Fe, which is called austenite. Unstable below 910°C, becoming δ-ferrite at 1395°C. Maximum carbon solubility is about 2.1% at 1147°C. Austenite is soft and ductile and does not have magnetic properties;
- The alpha ferrite phase (α-Fe) is a solid solution of C in α-Fe with a bcc lattice. It is considered the most stable form of iron at room temperature. The maximum solubility of carbon is about 0.02% at 727 °C. Softer than austenite, magnetic.
Austenite in steels
Austenite is always present in stainless steels, which contain from 16 to 26 percent chromium and up to 35 percent nickel. Austenitic steels, in addition to high corrosion resistance, are not hardened by heat treatment and are non-magnetic.
The forms of existence of cementite are important for determining the corrosion resistance of steels. Cementite (Fe3C) has been shown to increase corrosion rates, with this effect being more pronounced when it forms a coherent network on the surface. In normalized steels, cementite forms a coherent network, but in tempered martensite it does not. Therefore, cementite affects the corrosion rate of normalized but not hardened steel.
In general, the corrosion rate of carbon steel decreases with increasing chromium content due to the formation of protective chromium oxide. However, when chromium combines with carbon to form chromium carbide, the positive effect of chromium is lost.
The influence of the microstructure of various low alloy steels shown in this model only applies to conditions in which protective films do not form. Moreover, the formation of a carbide network on the surface of normalized steel is a time-dependent process.
Nodal critical points
An iron-carbon diagram with explanations makes it easier to study the kinematics of the formation of various microstructures. Phase diagrams help metallurgists understand which phases are thermodynamically stable, metastable, or unstable. Using them, you can select the appropriate elements for alloying steel. Phase diagrams also show us how to solve problems such as intergranular corrosion, hot corrosion, hydrogen damage, etc.
The characteristic point on the diagram is the eutectic point - the place where several phases meet. In the iron-carbon diagram, the eutectic point is where lines A1, A3 and ACM intersect. The formation of these points is random.
At these points, eutectic reactions occur, in which the liquid phase solidifies and turns into a mixture of two solid phases. This occurs when a liquid alloy of eutectic composition is cooled to its eutectic temperature.
The alloys formed at this stage are known as eutectic alloys. To the left of this point the alloys are called hypereutectic, and to the right - hypereutectic.
Carbon - element characteristics and chemical properties
Characteristics of carbon.
Properties of simple substances and compounds Carbon (C) is a typical non-metal;
in the periodic table it is in the 2nd period of group IV, the main subgroup. Serial number 6, Ar = 12.011 amu, nuclear charge +6. Physical properties: carbon forms many allotropic modifications: diamond is one of the hardest substances, graphite, coal, soot .
A carbon atom has 6 electrons: 1s 2 2s 2 2p 2. The last two electrons are located in separate p-orbitals and are unpaired. In principle, this pair could occupy the same orbital, but in this case the interelectron repulsion greatly increases. For this reason, one of them occupies 2px, and the other, either 2py or 2pz orbitals.
The difference in the energy of the s- and p-sublevels of the outer layer is small, so the atom quite easily goes into an excited state, in which one of the two electrons from the 2s orbital passes to the free 2p. A valence state appears with the configuration 1s 2 2s 1 2px 1 2py 1 2pz 1 . It is this state of the carbon atom that is characteristic of the diamond lattice—tetrahedral spatial arrangement of hybrid orbitals, identical length and energy of bonds.
During sp hybridization, the s and p orbitals overlap. An angle of 180° arises between the two equivalent hybrid orbitals that are formed, while the two p-orbitals of each atom remain unchanged.
Allotropy of carbon. Diamond and graphite
Meaning of chart lines
Boundaries that intersect each other mark specific areas on the diagram. Within each zone there can be a single phase or two phases. A phase transition occurs at the boundary. These regions are phase fields, they indicate the phases present for a particular alloy composition and temperature.
There are several characteristic lines on the diagram, designated A1, A2, A3, A4 and ACM. When the temperature of the metal increases or decreases, a phase transition occurs at these boundaries. Typically, when an alloy is heated, its temperature rises, but along these lines, heating causes the structure to rearrange itself into a different phase, and thus the temperature stops increasing until the phase completely changes. This process is called thermal shutdown.
The alloy steel elements—nickel, manganese, chromium, and molybdenum—affect the position of these boundaries on the phase diagram. Borders can move in any direction depending on the element used. For example, in the phase diagram of iron and carbon, adding nickel lowers the A3 limit, and adding chromium increases it.