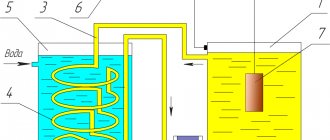
Purpose of nickel-cobalt alloy coating
The nickel-cobalt alloy has a shiny silver-gray color. It is used:
- The main thing is as hard magnetic coatings for recording information in memory elements or recording sound. Simply put, the coating is a carrier of information;
- As protection against external magnetic fields;
- In the manufacture of magnetic circuits;
- In the manufacture of matrices for casting and pressing plastic products.
The process of electrolytic deposition of nickel-cobalt alloy has not been fully studied, but has received significant industrial application.
Nickel-cobalt electroplating designation: N-Co
Deposits in Russia and the world
More than 400 deposits produce nickel ores around the world. The largest domestic deposits are located in the following areas:
- Taimyr district - Oktyabrskoye and Talnakhskoye.
- Murmansk region - Zhdanovskoye.
- Chelyabinsk region - Sakharinskoe.
- Ural - Serovskoe.
- Orenburg region - Buruktalskoe.
Smaller ones are present in Karelia, the Voronezh region, on the territory of the Republic of Tyva, in the Amur region and the Kamchatka Territory.
There are significant deposits abroad:
- in Canada, Cuba, South Africa,
- in Ukraine, Albania and Greece,
- in Indonesia, the Philippines, Australia and New Caledonia.
List of locations of the largest cobalt deposits by country of the world:
- Bou Azzer, Morocco.
- Qinghai, China.
- Ramu, Papua New Guinea.
- Voisey's Bay Mine, Sudbury Area Mine, Nunavik Mine, Canada.
- AdlayCagdianaoTandawaProject, Philippines.
- Flounder, Mount Keith, Marrin, Australia.
- Moa region, Cuba.
- Altai Republic, Russia.
- Katanga Province, Congo.
- Insizwa, South Africa.
Electrolytes and coating deposition modes
The potentials of nickel and cobalt in solutions differ slightly from each other, so precipitation of the alloy is not particularly problematic.
The composition of the alloy and deposition modes greatly influence the value of the so-called coercive force. The maximum value of this value is achieved when the nickel content in the alloy is 30%.
Coating deposition is possible from chloride, sulfate, sulfate-chloride, sulfamate and fluoroborate electrolytes.
Table 1 - Influence of the electrolyte on the magnitude of the coercive force
| Electrolyte for alloy deposition | Magnitude of coercive force |
| Sulfamate | 40·103 A/m |
| Sulfate | 24·103 A/m |
| Chloride | 17·103 A/m |
| Fluoroborate | 16·103 A/m |
The composition of the electrolyte directly affects the composition of the resulting alloy. With a ratio of Ni2+ and Co2+ of 1:1, the nickel content in the alloy will be 5%. By increasing the nickel content to 5:1, its content will increase to 40%.
Table 2 - Example of optimal sulfuric acid electrolyte and modes of deposition of nickel-cobalt alloy
| Electrolyte composition and modes | Parameter value |
| Nickel sulfate NiSO4 7H2O | 130-140 g/l |
| Cobalt sulfate CoSO4 7H2O | 110-120 g/l |
| Boric acid H3BO3 | 20-30 g/l |
| Potassium chloride KCL | 10-15 g/l |
| pH | 4-5 |
| Current Density | 1-2 A/dm2 |
| Temperature | 40-50 °C |
| Alloy | 14-35% Ni and 86-65% Co |
When depositing an alloy, it is worth considering that the anodes must be powered individually. If you want to get an alloy with 40% nickel, then you need to pass 40% of the total current only through the nickel anodes. The electrochemical equivalents of nickel and cobalt are close.
Side subgroup of the 8th group
(or, according to modern nomenclature, groups 8, 9 and 10)
| This includes nine elements: | 8 | 9 | 10 |
| As already discussed, towards the end of the d-series the change in properties slows down. | Fe | Co | Ni |
| Over the period, the value of the highest oxidation state increases, but its stability | Ru | Rh | Pd |
| decreases and lower oxidation states are stabilized. Elements | Os | Ir | Pt |
groups 9 and 10 do not exhibit oxidation states 9 and 10, and the same oxidation states are most stable. As a result, the similarity of elements horizontally becomes greater than vertically. Therefore, it is more convenient to consider the Fe-Co-Ni triad together, and then consider the 6 remaining elements, united under the name “platinum metals”. Note that in the textbook by F. Cotton and J. Wilkinson, this principle is maintained throughout: the d-elements of the fourth period are considered first, and then all the others.
Nickel-iron alloy plating
The coating is used as a soft magnetic coating (coercive force less than 1000 A/m). Used as magnetic protection against external fields and in magnetic video recording heads.
The composition of the electrolyte also plays a decisive role. To obtain a coating with the required minimum coercive force, a nickel content in the alloy is required - 80%.
To obtain the coating, it is recommended to use sulfamate and fluoroborate electrolytes. In them, coatings are deposited with low internal stresses, which is especially important when depositing thick alloys. Fluorocarbon electrolyte is highly stable.
Nickel ore processing technology
The technology for processing nickel ores is complex and multi-stage. Sometimes some of the raw materials have to be returned to previous stages of the process. To a large extent, it is determined by the percentage of the desired mineral in the feedstock.
So:
- nickel-rich ores (over 1% of the composition) are immediately sent for smelting;
- the poorer - ordinary people - are enriched;
- silicate ferruginous ores undergo hydrometallurgical processing;
- silicate magnesium ores are used for pyrometallurgical processing;
- There are also complex combined schemes with which oxidized and mixed ores are processed.
To nickel
In order to obtain nickel from ore, the initial raw material goes through a number of processing stages:
- First of all, the ore is cleaned of moisture and rottenness. To do this, it is crushed, then dried and sintered in ovens.
- The fluxes and gypsum obtained as a result of the first stage are diluted with coke and the entire resulting mass is melted into matte (an intermediate metallurgical product).
- As a result of smelting, matte and slag are formed. The slag is sent to the dump. And the matte is blown in a convector.
- After this, white nickel matte and again slag are obtained, part of which is again sent for smelting, and the other part is used to produce carbon monoxide.
- The white mass, containing a significant amount of nickel, is again crushed and ground, and then sent for firing.
- Nickel oxide is reduced using charcoal.
- The final stage in obtaining the desired chemical element Ni is electrolytic refining.
Nickel
To cobalt
To obtain cobalt from nickel ores, they are first dissolved with sulfuric acid, ammonia or water. Pyrometallurgical processes are sometimes used.
Cobalt hydroxides are then obtained using chlorine compounds or cobalt is isolated at cathodes using electrolysis using electrolysis. To obtain high-purity metal, the solution is first thoroughly cleaned of impurities: copper, iron, lead and nickel.
Study of iron-cobalt alloys with high magnetic saturation (article)
INTRODUCTION
In modern instrument making, alloys with high magnetic saturation are often used. Among the alloys with the highest saturation is iron). The only alloying element that increases its saturation is cobalt, and, therefore, iron and cobalt alloys have the highest magnetic saturation. The magnetic saturation curve of iron-cobalt alloys according to A. Kussman, B. Sharnov and A. Schulze [1] is shown in Fig. 1, from which it follows that it has a gentle maximum in the range of 30–50% Co. Magnetic saturation in this range can reach 24500 gus; these alloys are used in technology.
Alloys containing 40-60°/o Co, when cooled even at high speeds, acquire an ordered structure (2.3) and become brittle. In order for them to be deformed in a cold state, 1-2% vanadium, chromium or manganese are introduced into their composition, which apparently reduce the degree of ordering, so that after hardening it is possible to obtain a fairly plastic material.
In this work, a study was carried out of the dependence of magnetic properties (initial permeability and coercive force), as well as hardness, allotropic transformation temperature, microstructure of alloys containing 50% Co and 1.5% V, on heat treatment and cold deformation regimes with the aim of determining optimal conditions for production technology and heat treatment of alloys.
The alloys were smelted in a 35 kg high-frequency induction furnace and cast into 17 kg ingots, which were forged and hot and cold rolled.
State diagrams of pure iron-cobalt alloys, as well as alloys with vanadium additives, are known [2, 3, 4]. However, in order to resolve the issue of heat treatment temperatures during the production of the alloy and its final heat treatment, it was necessary to determine the critical points of the alloys smelted under the conditions of the experimental plant of the Central Research Institute of Chemistry and to find out how the temperature of allotropic transformations changes with small changes in the chemical composition.
Critical points were determined using a Leitz-type dilatometer at a heating and cooling rate of 3°C/min. The obtained data are shown in the table.
Critical points of alloys (50% Co) depending on vanadium content
| Content vanadium% | Temperature, °C | |
| the beginning of transformation upon heating | end of transformation upon cooling | |
| 0,00* | 980 | 980 |
| 1,38 | 915 | 870 |
| 1,68 | 885 | 810 |
| 1,95 | 720 | 600 |
| 4,0 | 700 | 555 |
*According to literature data.
Pure iron-cobalt alloys containing about 50% Co crystallize into a face-centered lattice
(γ-phase) At 980° they undergo a transformation to form the α-phase, which has a body-centered lattice. The γ->α transformation occurs in a narrow temperature range and has almost no hysteresis [2, 3]. When vanadium is introduced into an alloy, the transformation temperature decreases [4] and at the same time the transformation hysteresis increases.
When the content is above 4% V, the transformation upon cooling descends to the temperature range below 600° and acquires a martensitic character. Thus, the effect of vanadium on the Fe-Co alloy is opposite to the effect of vanadium on iron, where it sharply limits the range of γ-alloys.
Since alloys with a γ-phase lattice are non-magnetic, along with the allotropic α-γ transformation, the possibility of using the ferromagnetism of the α-phase, the Curie point of which lies above the temperature of the allotropic transformation, is lost. Therefore, in cases where it is necessary to have ferromagnetism up to the highest temperatures, it is better to use iron-cobalt alloys that do not contain vanadium.
upon cooling in air, grain boundaries are clearly revealed; the grains have more regular outlines. After slow cooling, a characteristic structure in the form of a network is formed in the grains (Fig. 2), often having a pronounced orientation along the directions of the crystalline planes.
The same structure is observed in samples slowly cooled from a temperature of 860°, which lies below the allotropic transformation temperature, but above the ordering temperature. The formation of this structure may be associated with ordering of the alloy upon cooling. Quenching at 860° in water fixes the grains grown as a result of the recrystallization process. The grains show emerging grid lines, typical of slow cooling. Apparently, quenching from 860° in water does not completely prevent the ordering process, which is confirmed by some brittleness of the alloy after quenching.
To determine the intermediate heat treatment temperature during cold rolling, it was necessary to study the recrystallization process of the cold-worked alloy. To do this, we studied the microstructure and hardness of samples with a thickness of 0.2 and 0.8 mm, subjected to cold rolling with a reduction degree of 67 and 17%, and then heated to various temperatures in the range of 200-1000° for 2 hours. and subsequent slow cooling with the oven. The results of hardness measurements after heating to different temperatures are shown in Fig. 4 (curves 1 and 2). Samples rolled with a higher degree of compression have greater hardness. This difference persists up to 700°, above which intense recrystallization processes lead to a leveling of hardness. The hardness change curves (curves 1 and 2) reveal an interesting phenomenon - an increase in the hardness of cold-rolled samples after heating them to 200-600°. The greatest increase in hardness, reaching 100-150 Vickers units, occurs after tempering at 400-500°. If the samples are pre-hardened at 800° (above the ordering temperature), then the hardness does not increase after heating to different temperatures (curves 3 and 4).
The recrystallization process begins at 650° and proceeds intensively at 700° and higher temperatures. This is also confirmed by microstructure studies. Based on the experiments carried out, quenching from 860° in cold water was adopted as an intermediate heat treatment.
The study revealed that slightly different heat treatment regimes for slabs and cold-rolled sheets should be adopted.
In Fig. Figure 5 shows the dependence of the initial permeability of samples 17 mm thick on the annealing temperature. The samples were placed in tubes, and the spaces between the samples and the walls of the tube were tightly packed with calcined asbestos. The tubes were covered with a refractory mass and loaded into the furnace.
As can be seen from Fig. 5, the best values of the initial permeability correspond to annealing at 1100-1200°. Increasing the annealing temperature to 1300° leads to a decrease in the initial permeability due to significant oxidation of the sample surface.
Exposure - for 5 hours. at the annealing temperature, as experiments have shown, is quite sufficient. With longer exposures, the initial permeability does not increase, and with shorter exposures it does not yet reach its maximum.
The cooling rate, especially from 800°, has a significant influence on the magnetic properties. To achieve optimal values of initial permeability, the cooling rate from the annealing temperature (1100°) should be no more than 50–100° C/hour.
Samples 0.2 mm thick were annealed in a hydrogen atmosphere in an electric furnace with automatic temperature control and recording. Frames with rods fixed in the walls were loaded into the furnace, onto which the samples were suspended so that each of them was freely washed by a hydrogen flow.
In Fig. Figure 6 shows curves expressing the dependence of the initial permeability of thin samples (0.2 mm) of two melts, rolled with a compression of 60%! on the annealing temperature. The duration of exposure at the annealing temperature was 5 hours, cooling from the annealing temperature to 300° was carried out at a rate of 50°C/hour. The initial permeability curves have a maximum after annealing at 850° (heat 1407) and at 900° (heat 1406).
The increase in initial permeability with increasing annealing temperature is apparently associated with stress relief and recrystallization of the metal at 750-800°, accompanied by grain growth. The grain size is determined by the annealing temperature: the higher the temperature, the larger the grain. However, the increase in annealing temperature is limited by the recrystallization temperature, since grain refinement occurs during the recrystallization process.
As revealed by dilatometric tests, samples of heat 1406 have a higher phase recrystallization temperature than samples of heat 1407. Therefore, it was possible to obtain higher permeability at a higher annealing temperature. The holding time during annealing should be 3-5 hours.
In Fig. Figure 7 presents the results of a study of the effect of cooling at different rates on the properties of an alloy rolled with a reduction degree of 50%. When the cooling rate decreases from 100 to 20° C/hour, the initial permeability approximately doubles
CONCLUSIONS.
- The hardness, microstructure, critical points and magnetic properties of alloys containing 50% Co and about 2% V have been studied. Vanadium significantly changes the critical points of alloys of the Fe - Co system, reducing the temperature of the α-> γ transformation. In cases where the highest temperature of magnetic transformation is required, as well as high magnetic saturation, it is preferable to use an alloy without vanadium.
- The microstructure of alloy samples slowly cooled from high temperature has a characteristic network in the grains, probably associated with the phenomenon of ordering.
- The hardness of cold-rolled samples increases significantly when heated to 400-600°; with a further increase in temperature, a decrease in hardness occurs due to the development of the recrystallization process.
- Annealing of samples cut from forged blanks to obtain the highest initial permeability should be carried out in hydrogen, vacuum or asbestos at 1100° for 5-10 hours. followed by slow cooling (50-100°C/hour). Annealing of samples subjected to pre-cold deformation is carried out in hydrogen or vacuum at 850-900°.
- The degree of compression during cold deformation has a significant influence on the initial permeability. The optimal compression ratio seems to be 60-70%.