Physics Glossary
|
Strength of solids in a broad sense
— the ability of solids to resist destruction (separation into parts), as well as irreversible changes in shape (plastic deformation) under the influence of external loads.
Strength of solids in the narrow sense
- resistance to destruction. Depending on the material, the type of stress state (tension, compression, bending, etc.) and operating conditions (temperature, load duration, etc.), various measures of the strength of solids (yield strength, tensile strength, fatigue limit) are used in technology etc.).
The destruction of a solid body is a complex process that depends on many factors, therefore the values that determine the strength of solid bodies are conditional.
Rice. 1. Dependence of the force of interaction between two atoms on the distance between them.
Physical nature of strength
The strength of solids is ultimately determined by the interaction forces between the atoms or ions that make up the body. For example, the strength of interaction between two neighboring atoms (if we neglect the influence of surrounding atoms) depends only on the distance between them (Fig. 1). At an equilibrium distance r0 ~ 0.1 nm (1), this force is zero. At smaller distances the force is positive and the atoms repel, at larger distances they attract. On critical distance force of attraction in abs. the value is maximum and equal to FT. For example, if when stretching a cylinder-rich. rod with cross section S0, the acting force P directed along its axis is such that the external force per given pair of atoms. force exceeds max. force of attraction FT, then the atoms move away from each other without hindrance. However, for a body to collapse along a certain surface, it is necessary that all pairs of atoms located on both sides of the surface in question experience a force exceeding FT. The voltage corresponding to the force F t is called. theoretical tensile strength s t (sT0.1 E, where E is Young’s modulus). However, in practice, destruction is observed under load P*, which corresponds to a voltage s = P*/S 100-1000 times less than s t· Discrepancy between the theoretical. P. t. t. with real is explained by inhomogeneities in the structure of the body (grain boundaries in polycrystalline material, foreign inclusions, etc.), due to which the load P is distributed unevenly over the cross section of the body.
Mechanism of destruction
If on a surface area of small dimensions (but significantly larger than the cross section of one atom) the local stress is greater than s t, a rupture will occur along this area. The edges of the rupture will diverge to a distance greater than rк, at which point the interatomic forces are already small, and a microcrack will form (Fig. 2). The nucleation of microcracks at stress below st is promoted by thermal. fluctuations.
Rice. 2. Griffith crack; The area in which stress is relieved is shaded. Arrows indicate the direction of voltage.
Local stresses are especially high at the edge of the formed crack, where stress concentration occurs, and the larger the crack size, the greater they are. If this size is more than a certain critical. rс, the atoms at the edge of the crack are subject to a stress exceeding st, and the crack grows further along the entire cross-section of the body at high speed - destruction occurs. The value of rc is determined from the condition that the elastic energy of the material released during crack growth covers the energy costs for the formation of a new crack surface: (where g is the energy per unit surface of the material). Before the increasing ext. the force will reach the value necessary for destruction, dep. groups of atoms, especially those that are part of defects in crystals, usually experience rearrangements, during which local stresses decrease (“relax”). As a result, an irreversible change in the shape of the body occurs - plasticity. deformation; it is also facilitated by thermal. fluctuations. Fracture is always preceded by greater or lesser plasticity. deformation. Therefore, when estimating rc, the energy g must include the work of the plastic. deformation ur. If plastic the deformation is large not only near the fracture surface, but also in the volume of the body, then the fracture is viscous. Destruction without noticeable traces of plastic. deformations called fragile. The nature of destruction is manifested in the structure of the fracture surface. In crystalline In bodies, brittle fracture corresponds to crystallographic chipping. cleavage planes, viscous - merging of microvoids and sliding. At low temperatures, destruction is predominant. brittle, at high - viscous. The rate of transition from ductile to brittle fracture is called. critical cold brittleness temperature.
Since fracture is the process of nucleation and growth of cracks and pores, it is characterized by the speed or time from the moment the load is applied to the moment of rupture, i.e., the durability of the material. Research of many crystalline and amorphous bodies showed that in a wide range of temperatures T and stresses s applied to the sample, tensile durability is determined by the relation
where approx. equal to the period of thermal vibrations of atoms in a solid (10-12 s), the energy U0 is close to the sublimation energy of the material, activation. volume V is usually several. thousand atomic volumes and depends on the structure of the material formed during the preliminary thermal process. and mechanical processing and during loading. At low temp-pax, the durability drops very sharply with increasing stress, so that for any value important for practice, there is an almost constant limiting value of stress above which the sample is destroyed almost instantly, and below it it lives indefinitely. This value of s0 can be considered the strength limit (table).
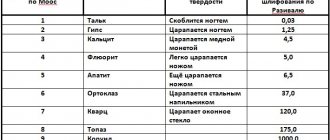
Tensile strength values
kgf/mm2 (1 kgf/mm2=10 MN/m2)
| s0 | s0/E | |
| Graphite (filamentary crystal) | 2400 | 0,024 |
| Sapphire (threaded crystal) | 1500 | 0,028 |
| Iron (whisker) | 1300 | 0,044 |
| High Carbon Steel Drawn Wire | 420 | 0,02 |
| Tungsten drawn wire | 380 | 0,009 |
| Fiberglass | 360 | 0,035 |
| Mild steel | 60 | 0,003 |
| Nylon | 50 |
Time is spent waiting for thermal fluctuations. the initiation of microcracks and their growth to critical. size . When a stress s is applied to a sample, it deforms first elastically, then plastically, near the structural inhomogeneities that were present in the initial state or that arose during plasticity. deformation, large local stresses are formed (for example, in crystals - as a result of the accumulation of dislocations). Microcracks originate in these places. Their concentration can be very high (for example, in some polymers there are up to 1015 cracks per 1 cm3). However, their sizes, determined by the scale of structural heterogeneities, are significantly smaller. Under the post. under stress, the size and concentration of cracks grow slowly and the body does not collapse until by chance (for example, as a result of the sequential merging of closely spaced adjacent cracks) one of them grows to critical. size. Therefore, when creating durable materials, care should be taken not so much to ensure that cracks do not appear, but rather to ensure that they do not grow.
Random distribution of structural inhomogeneities over the volume of the sample, in size and in degree of strength and the random nature of the term. Fluctuations lead to a scatter in the durability values (as well as the P.T.T. limit) when testing identical samples at given values of T. The greater the probability of encountering a “weak” point in a sample, the larger its volume. Therefore, the PT (breaking stress) of small samples (for example, thin threads) is higher than that of large ones made of the same material (the so-called scale effect). Areas with increased stress, where microcracks nucleate more easily, are more common near the surface (protrusions, scratches). Therefore, surface polishing and protective coatings increase P. t. t. On the contrary, in aggressive environments P. t. t. is reduced.
Yield strength
The most interesting parameter is the yield strength. At the beginning of the test, when the sample begins to stretch, the deformations in its structure are reversible. That is, if you stop stretching before a certain point, the sample under study will return to its previous state due to elastic deformation.
However, after reaching the “point of no return,” the metal can no longer elastically return to its original dimensions—irreversible plastic deformation begins. The voltage at which this occurs is recorded by equipment, and is subsequently taken into account when describing the strength characteristics of the sample.
It is interesting that when calculating load-bearing structures, engineers mainly rely on the yield strength, and not on the tensile strength of the metal.
Mechanical properties of metals
Strength of metals
Strength
- the property of solids that resists destruction, as well as irreversible changes in shape. The main indicator of strength is the temporary resistance, determined at the rupture of a cylindrical sample that has been previously annealed.
Based on their strength, metals can be divided into the following groups:
weak metals
- (temporary resistance does not exceed 50 MPa) - tin, lead, bismuth, as well as soft alkali metals.
durable metals
- (from 50 to 500 MPa) - magnesium, aluminum, copper, iron, titanium and other metals that form the basis of the most important structural alloys
high strength metals
— (more than 500 MPa) — molybdenum, tungsten, niobium, etc.
The concept of strength does not apply to mercury, since it is a liquid.
The tensile strength of metals is indicated in Table 10.
Table 10. Strength of metals
| Metal | Tensile strength, MPa | Metal | Tensile strength, MPa |
| Titanium | 580 | Zinc | 120-140 |
| Iron | 200-300 | Aluminum | 80-120 |
| Copper | 200-250 | Gold | 120 |
| Magnesium | 120-200 | Tin | 27 |
| Silver | 150 | Lead | 18 |
Plasticity of metals
Plastic
- the property of solids to retain part of the deformation when the loads that caused them are removed. As an indicator of ductility, relative elongation is selectively determined by the same tests as tensile strength.
According to the degree of ductility, metals are usually divided as follows:
highly plastic metals
- (relative elongation exceeds 40%) - metals that form the basis of most structural alloys (aluminum, copper, iron, titanium, lead) and “light” metals (sodium, potassium, rubidium, etc.)
ductile metals
- (relative elongation ranges between 3% and 40%) - magnesium, zinc, molybdenum, tungsten, bismuth, etc. (the most extensive group)
brittle metals
- (relative elongation less than 3%) - chromium, manganese, colbate, antimony.
High purification of brittle metals slightly increases ductility. Alloys obtained from them are almost impossible to process under pressure. Industrial products from them are often produced by casting. The relative elongation of metals is characterized in Table 11.
Table 11. Plasticity of metals
| Metal | Relative extension, % | Metal | Relative extension, % |
| Gold | 65 | Titanium | 50 |
| Silver | 65 | Tin | 40 |
| Lead | 65 | Aluminum | 30-40 |
| Copper | 50-60 | Zinc | 30 |
| Iron | 40-50 | Magnesium | 10-22 |
Hardness
Hardness
- a characteristic of a material that reflects its strength and ductility, determined by indenting a ball (Brinell method) or a prism (Vickers method). A quantitative assessment of hardness is the hardness number HB, equal to the ratio of the load (N) to the surface area of the print (mm2).
The Brinell hardness values of metals are given in Table 12.
Table 12. Hardness of metals
| Metal | NV | Metal | NV |
| Titanium | 160 | Aluminum | 16-25 |
| Iron | 70-80 | Silver | 25 |
| Magnesium | 30-40 | Gold | 18 |
| Copper | 40 | Tin | 5 |
| Zinc | 33 | Lead | 4 |
Modulus of longitudinal elasticity
Modulus of longitudinal elasticity, Young's modulus, E
— determines the hardness of the metal, i.e. the intensity of the increase in stress as the elasticity of the deformation increases.
Table 13. Young's modulus of metals at 20 oC
| Metal | E * 10-5, MPa | Metal | E * 10-5, MPa |
| Iron | 2,17 | Gold | 0,83 |
| Zinc | 1,30 | Aluminum | 0,72 |
| Copper | 1,25 | Tin | 0,55 |
| Titanium | 1,08 | Magnesium | 0,45 |
| Silver | 0,83 | Lead | 0,18 |
Strength Literature
- Gul V. E., Structure and strength of polymers, 3rd ed., M., 1978;
- Destruction, trans. from English, vol. 1, M., 1973;
- Regel V.R., Slutsker A.I., Tomashevsky E.E., Kinetic nature of the strength of solids, M., 1974.
to the library to the table of contents FAQ on ethereal physics TOEE CHPP TPOI
Did you know,
that relativism (SRT and GTR) is not a true science? — True science necessarily relies on causality and the laws of nature given to us in physical phenomena (facts). In contrast, STR and GR are built on axiomatic postulates, that is, fundamentally unprovable dogmas in which the followers of these teachings are obliged to believe. That is, relativism is a form of religion, a cult, inflated by the political machine of the mythical authority of Einstein and his faithful followers, elevated to the rank of saints of relativistic physics. Read more in the FAQ on ethereal physics.
The strongest metal alloy in the world
Platinum + gold - the world's hardest metal alloy
And straight to the heart of the scientific achievement - scientists from Sandia National Laboratory in the USA have developed a new metal alloy and called it the strongest alloy ever created by scientists in laboratories around the world. The newly developed material, made from a combination of platinum and gold, is estimated to be 100 times stronger than high-strength steel, making it the first metal alloy in the same class as diamond surfaces.
Plasticity characteristics
Relative elongation is the difference between the initial and final length of a tensile sample, showing the ability of the metal to plastically deform until failure. Metals with the same tensile strength may have different elongations. For example, for malleable cast iron grade KCh50-5 this figure does not exceed 5%, and for structural steel 09G2S it reaches 20% with a tensile strength equal to 490 MPa for both materials.
The metallurgical industry always strives to create high-strength metal materials without loss of ductility, selecting optimal chemical compositions of steel, and improving production technologies. To achieve high mechanical properties, while maintaining the same composition and volume of the product, unique modes of smelting, mechanical, thermal, chemical-thermal treatment are selected to create a homogeneous, fine-grained, clean and defect-free steel structure.