History of discovery
A new era in the development of chemistry began in the 17th century. During this period, a chemist from England named Gro made a discovery that brought scientists closer to the isolation of magnesium. In 1695, during the evaporation of Epsom mineral water, he received bitter salt, which had laxative properties.
A few years later, research showed that the interaction of the substance with soda and potassium carbonate produces a white, loose powder. The same result was obtained during the calcination of a mineral that was found near the city of Magnesia in Greece. Because of this similarity, the salt came to be called white magnesia.
Magnesium itself was first obtained by Humphry Davy in 1808. The scientist carried out electrolysis of white magnesia, to which he added a small amount of water and mercuric oxide. This reaction resulted in the formation of an amalgam of metallic substance. The resulting metal, after removal, was called “magnesium.”
It should be noted that the reagent had various impurities. The pure element was discovered in 1829 by the French chemist Antoine Alexandre Brutus Bussy.
Valency Mg
Magnesium atoms in compounds exhibit valency II.
The valency of magnesium characterizes the ability of the Mg atom to form chemical bonds. Valence follows from the structure of the electron shell of the atom; electrons involved in the formation of chemical compounds are called valence electrons. A more extensive definition of valence is:
The number of chemical bonds by which a given atom is connected to other atoms
Valence has no sign.
Definition and physical characteristics
The substance is a representative of group II of the periodic table of chemical elements.
It can be characterized as follows:
- Magnesium is usually denoted as Mg.
- The atomic number of the substance is 12.
- The molar mass of the element is 24.305 atomic units.
- The nuclear charge is 12.
- Each atom has 12 electrons.
- The crystal lattice of the substance has the α-form Ca face-centered cubic. It is stable at normal temperatures.
- Density - 1.738 g/cm³.
- Metal melts at a temperature of 650 °C.
- The substance boils at 1090 °C.
- A pure element is characterized by plasticity. It can be easily pressed, rolled and cut.
The reagent in its compact state is a shiny silvery-white metal. When exposed to air, it fades as a film of oxide forms on the surface.
The metal reagent burns with a dazzling white flame . The speed at which the substance flares up is many times greater than the speed at which the hand is pulled back. Because of this physical property of magnesium, the person igniting the element must follow all required safety regulations. Otherwise, he will receive a serious skin burn. To observe the combustion process, you should use dark glasses or glass. Failure to take these precautions increases the risk of retinal burns, which can lead to temporary blindness.
Note:
201* The range of atomic mass values is indicated due to the varying abundance of isotopes of a given element in nature.
205* The empirical radius of the magnesium atom according to [1] is 160 pm.
206* The covalent radius of magnesium according to [1] and [3] is 141±7 pm and 136 pm, respectively.
407* The specific heat of fusion (enthalpy of fusion ΔHmel) of magnesium according to [3] is 9.20 kJ/mol.
408* The specific heat of evaporation (enthalpy of boiling ΔHboiling) of magnesium according to [3] is 131.8 kJ/mol.
410* The molar heat capacity of magnesium according to [3] is 24.90 J/(K mol).
Chemical properties
All stable compounds of the element have a valence of two, and the electronic formula of magnesium or the schematic structure of its atom is: 1s2 2s2 2p6 3s2.
Even when heated to 350 °C, compact magnesium undergoes minor oxidation because it is covered with an oxide film. The reagent burns at a temperature of 600 to 650 °C, and oxide and nitrite of the element are formed. Nitrite can also be obtained by heating the substance to 500 °C in a nitrogen environment.
The element belongs to active substances. In addition, the following chemical properties of magnesium are distinguished:
- Does not react with cold water that is not saturated with air.
- Gradually displaces hydrogen from boiling water.
- Reacts with water vapor at temperatures not lower than 400 °C.
- In liquid form, it releases hydrogen from moist air, absorbing it.
- As the element solidifies, it almost completely removes hydrogen.
- When the hydrogen atmosphere is heated to 400−500 °C, the substance reacts with it, forming magnesium hydride.
- Displaces most of the metal elements from solutions of their salts formed on the basis of water.
- In cold conditions, it combines with moist chlorine to form magnesium chloride.
- When heated, it reacts with halogens.
- Most of the element's salts are highly soluble in water.
- Upon contact with caustic alkalis, a precipitate formed from solutions of magnesium salts forms.
- At low temperatures it does not react with aqueous alkaline solutions, but is susceptible to dissolution of alkali metal reagents and ammonium salts in bicarbonates.
- Insoluble in concentrated sulfuric acid.
- It is protected from dissolution in hydrofluoric acid by a film of stable fluoride.
- Interacts with dilute mineral acidic compounds at low temperatures.
The reagent is a strong reducing agent . Heated magnesium displaces some metals and non-metals from oxides and halides. There are many organometallic compounds of the substance, which make it one of the most important elements of organic synthesis.
The reagent easily creates alloys with many metals, and therefore the production of many very important light materials is based on it.
To which group of metals does iron and its alloys belong?
To which group of metals does iron and its alloys belong?
a) To refractory; b) To black; c) To diamagnetic materials; d) To metal with high specific strength
a) Refractory metals have a melting point higher than the melting point of iron
c) Iron and most of its alloys are pronounced ferromagnets
d) Structural materials are classified according to their specific strength. In addition, materials such as titanium alloys, beryllium and especially composites have higher specific strength than iron-based alloys
2. Which of the following metals (alloys) is considered ferrous?
a) Brass; b) Corrosion-resistant steel; c) Babbitt; d) Duralumin
a) Brasses are non-ferrous alloys whose main components are copper and zinc
c) Babbitts are non-ferrous anti-friction alloys based on tin or zinc
d) Duralumins are non-ferrous alloys based on aluminum
What are metals with a melting point higher than the melting point of titanium called?
a) Refractory; b) Noble; c) Black; d) Rare earth
b) Metals that are chemically inert (Rh, Pd, Ag, Os, Pt, Au) are classified as noble metals. They have t pl. both above (plate metals) and below (Ag, Au) t pl iron
c) Black includes iron and alloys based on it
d) Rare earth metals include gr. lanthanum-lanthanides (Ce, Pr, Nd, Sm) as well as yttrium (Y) and scandium (Sc) Most REMs have a tm lower than the tm of iron
What group of metals does tungsten belong to?
a) To actinides; b) To the noble; c) To rare earths; d) To refractory
a) Main difference The characteristic of actonoids is radioactivity. Natural tungsten radioact. has no isotopes
b) To the good. include Ag, Au, metals gr. platinum For them, maybe Tungsten copper is not included among these metals
c) In gr. REM includes lanthanides and similar yttrium and scandium Tungsten does not belong to lanthanides
Which of the following groups contains only refractory metals?
a) Nickel, aluminum; b) Titanium, sea anemone; c) Molybdenum, zirconium; d) Tungsten, iron
a) Nickel belongs to gr. iron, and aluminum - light metals. In addition, the t pl. of both metals is lower than the t pl. of iron
b) Ac refers to gr. uranium In addition, the t square of actinium (1050) is lower than the t square of iron
d) To tightness include metals with t pl above t pl Fe
6. Which group of metals (alloys) does magnesium belong to?
a) Low-melting; b) To the noble; c) To the lungs; d) To rare earths
a) t pl Mg is really low (650), but it has a character. sign, according to cat. it is classified as another group.
b) To the good. include Ag, Au, metals gr. platinum for them, maybe. Copper is included Magnesium is not among these metals
d) In gr. REMs include lanthanides and similar yttrium and scandium. Magnesium is not a lanthanide.
Which of the following groups contains only light metals?
a) Titanium, copper; b) Silver, chrome; c) Aluminum, tin; d) Magnesium, beryllium
a) Light metals include low-density metals, while copper is denser than iron
b) Silver belongs to the noble group, and chromium belongs to the refractory group. In addition, Ag is significantly higher in density than iron, Cr is only slightly inferior to it
c) Sn is low-melting (t pl = 232), moreover, the density of tin is only slightly inferior to iron
Which of the following groups contains only fusible metals?
a) Indium, magnesium; b) Tin, lead; c) Antimony, nickel; d) Zinc, cobalt
a) t pl In and Mg are really low (157 and 651), however, Mg, due to its low density (1.74 g/cm3), is classified as light
c) Only antimony has a low t pl (630), while for nickel it is quite high (1453). Nickel belongs to the metals of the iron group.
d) Only Zn (420) has a low tmelt, while for Co it is quite high (1493). Co belongs to the metals of the iron group.
What property of metals can be explained by the lack of directionality of interatomic bonds?
a) paramagnetism; b) electrical conductivity; c) anisotropy; d) high compactness
a) the magnetic properties of a material are determined by its electronic structure. Among metals there are not only paramagnets, but also diamagnets (Be, Zn, Cu, Ag)
b) Not only metals have high electrical conductivity, but, for example, graphite, a substance with directed interatomic bonds
c) anisotropy is characteristic of all crystalline bodies, including those with directed interatomic bonds
What is a domain?
a) unit of metal grain size; b) region of spontaneous magnetization of a ferromagnet; c) type of defect in the crystal structure; d) a section of metal grain with an undisturbed crystal lattice
a) the size of the metal grain is determined in units of length, or in points
c) domains are associated with the crystal structure of ferromagnets, but are not its defects
d) practically defect-free areas of the metal grain are called blocks of mosaic structure
To which group of metals does iron and its alloys belong?
a) To refractory; b) To black; c) To diamagnetic materials; d) To metal with high specific strength
a) Refractory metals have a melting point higher than the melting point of iron
c) Iron and most of its alloys are pronounced ferromagnets
d) Structural materials are classified according to their specific strength. In addition, materials such as titanium alloys, beryllium and especially composites have higher specific strength than iron-based alloys
2. Which of the following metals (alloys) is considered ferrous?
a) Brass; b) Corrosion-resistant steel; c) Babbitt; d) Duralumin
a) Brasses are non-ferrous alloys whose main components are copper and zinc
c) Babbitts are non-ferrous anti-friction alloys based on tin or zinc
d) Duralumins are non-ferrous alloys based on aluminum
General conditions for choosing a drainage system: The drainage system is selected depending on the nature of the protected area.
Mechanical retention of earth masses: Mechanical retention of earth masses on a slope is provided by buttress structures of various designs.
Papillary patterns of the fingers are a marker of athletic abilities: dermatoglyphic signs are formed at 3-5 months of pregnancy and do not change throughout life.
Source: cyberpedia.su
Being in nature
The earth is very rich in magnesium. Only six chemical reagents are found in nature more often than this substance. Most of the element is found in the planet's mantle, with less of it in the earth's crust. Most often it is found in basic rocks and granite. And the element is also found in various minerals formed by magma.
Pure magnesium is mainly extracted from three minerals:
- carnallite;
- dolomite;
- magnesite.
In Russia, the largest deposits of magnesite are located in the Middle Urals and the Orenburg region. Carnallite is mined near the city of Solikamsk; it should be noted that this deposit is the largest in the world. The most common mineral dolomite is found in the Moscow and Leningrad regions, as well as other regions of the country.
In the biological environment of the planet, magnesium compounds are constantly moving and changing. Only a small part of the element is retained in the cycle of substances occurring on the continents; a large amount of the reagent is carried away by rivers into the ocean. Despite the fact that the content of magnesium in sea water is second only to sodium, the liquid itself is not saturated with the element, and its salts do not precipitate in the open ocean.
The substance, in the composition of various compounds, accumulates in the salts remaining after water evaporates from the lagoons.
Buying powder or shavings
The easiest way to “get it” is to buy or order an element. Where can I get magnesium powder? You can find a store in your city or order powder or shavings delivered by mail to your home.
Today, magnesium is sold not only in bulk industrial containers. You can easily order it in a container of any packaging suitable for you. So, for example, 1 kg of magnesium powder will cost you, on average, 300 rubles.
The purchase can be made in the online stores “Alibaba”, “Ruskhim”, “Russian Metal”, “BVB Alliance”, Shilanet and others.
Receipt in industry
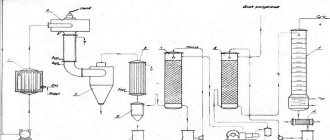
In industrial conditions, electrolysis of anhydrous chloride or anhydrous carnallite is most often used to obtain magnesium. The process goes like this:
- Electrolysis takes place at temperatures from 720 to 750 °C.
- As the elements are isolated, the composition of the bath is adjusted, part of the electrolyte is removed, and raw materials are added.
- The molten metal of interest floats to the surface and is regularly removed.
- The resulting substance contains many impurities. To clean the element, it undergoes refining in special furnaces under a layer of flux.
- The purified metal is poured into molds.
- The next purification involves sublimating the reagent several times under vacuum.
In addition to this method, metallothermic and carbon-thermal methods for producing magnesium are used in production. In the first case, briquettes from hot and decomposed dolomite are mixed with a reducing agent and heated in a vacuum at a temperature of 1300 °C. The resulting magnesium vapor forms condensate when the temperature drops to 400−500 °C. To clean the metal, use submerged arc melting or vacuum melting. The pure element is poured into molds.
When using the second method, briquettes consisting of coal and magnesium oxide are heated in electric ovens to 2100 °C. The metal that has turned into steam is distilled off and condensed.
The substance is also extracted from sea water. To do this, the raw materials are mixed in very large tanks with a suspension of calcium hydroxide, which is obtained by grinding sea shells. As a result of the chemical reaction that occurs, a special suspension is formed, which, after drying, becomes magnesium chloride. After this, the product is subjected to electrolytic processes.
In addition to sea water, water from some salt lakes can be used to distill magnesium. In the Russian Federation, such lakes are located in Crimea, the Volga region and other regions.
Application of the substance
Metallurgy consumes the largest amount of metal. On its basis, many alloys are created. The substance is often used in metallothermic processes to obtain rare metals, as well as those that are difficult to recover. A reagent is used for deoxidation and desulfurization of metallic substances. Various magnesium-based powder mixtures are used as lighting and incendiary materials.
Compounds containing the reagent have found wide application in various spheres of life. For example, in medicine, medications with magnesium can relieve patients from spasms and convulsions, calm the nerves, and so on.
EREPORT.RU
Magnesium was first discovered in the region of Thessaly, Greece, and named Magnesia. It is the third most abundant metallic element in the earth's crust, but is rarely found in its pure form due to the fact that it easily forms bonds with other elements. Magnesium metal was first obtained from its ore in 1808 in small quantities by Sir Humphry Davy, and industrial production first began in 1886 in Germany.
Magnesium is the lightest of all commonly used structural materials, with a density of 1.7 g/cm3 (106.13 lb/cu ft), about one-third lighter than aluminum and titanium, and one-quarter the density of steel. Despite this advantage, primary magnesium production in 2012 was 905 thousand tons, only 2.5% of primary aluminum production (45.2 million tons) and 0.06% of raw steel production (1546 million tons). However, the production volume of magnesium is greater than that of titanium (211 thousand tons).
Small additions of magnesium to aluminum impart fire resistance and strength. Magnesium's affinity with sulfur makes it indispensable in the production of certain grades of raw steel. With the help of magnesium, titanium metal is also reduced from titanium tetrachloride in the Kroll process, and very high-quality cast iron is also obtained. Together, these four areas accounted for 61% of magnesium consumption in 2012. Thus, despite its relative status as a minnow in the materials production mix, magnesium plays a central role in the manufacture and use of competing metal products.
Magnesium supplies
Global production of primary magnesium, according to Roskill estimates, increased from 499 thousand tons in 2002 to 905 thousand tons in 2012, a compound annual growth rate (CAGR) of 6.1%. Production of the primary metal magnesium is limited to ten countries.
China continues to dominate the production of primary magnesium metal. The country produced more than 730 thousand tons of metal in 2012, and its share that year accounted for more than 75% of the total supply. In China, however, there has been a shift in production. Abundant and cheap gas as a by-product of coke production prompted magnesium producers to turn their attention to Shaanxi province in search of higher profits. This has forced some traditional magnesium provinces to struggle with competitors, and overall the Chinese magnesium industry has a capacity utilization rate barely above 50%. In addition, there has been industrial consolidation in China, with eight Chinese manufacturers now among the top 10 global manufacturers.
Despite recent efforts by the Chinese government to consolidate the industry, most Chinese manufacturing capacity is still scattered in relatively small plants, with consolidation largely occurring at the corporate level. Eight Chinese companies are in the top 10 global suppliers in terms of capacity, which each exceeds 50 thousand tons per year, although only five of them produced more than 30 thousand tons in 2011, and one closed in 2012.
The number of companies with capacity below 50 kt, and production much less than 30 kt, is unknown, but Roskill estimates the number at around 50. Collectively, these small plants accounted for about a third of global capacity in 2012.
Source: Magnesium Metal: Global Industrial Markets and Outlook 2012, Roskill Information Services Ltd.
Despite several plant closures in the run-up to the 2008/09 recession, particularly in Canada, production in the US, Russia and Israel has since increased, albeit largely meeting demand from the growing titanium metal industry. Recycled magnesium production is more evenly distributed around the world, with the US still the number one recycler. New primary magnesium plants opened in Malaysia and South Korea in 2010, and Iran was due to follow suit in 2013. The expected launch of the Qinghai Salt Lake electrolytic plant in China, with a capacity of 100 thousand tons per year, may also change the balance of power in China in the short term.
The main producers of primary magnesium outside China are VSMPO-Avisma and the Solikamsk magnesium plant in Russia; US Magnesium in the USA; Dead Sea Magnesium in Israel; Ust-Kamenogorsk titanium-magnesium plant in Kazakhstan; Rima Industrial in Brazil; CVM Minerals in Malaysia; Magnohrom in Serbia; and POSCO in South Korea.
Recycled magnesium from recycled magnesium alloys, and as a component of recycled aluminum alloys, is an important source of supply, particularly in the US, where it accounts for about half of the total supply. It has much less meaning elsewhere. Global capacity and production of secondary magnesium (excluding aluminum alloys, which form a feedback loop) is estimated by Roskill at more than 200 thousand tons per year, with about 40% of capacity concentrated in the United States.
The majority of international magnesium trade is exports from China, which accounted for half of raw magnesium exports (99.8% of Mg raw magnesium exports in 2012. This material is mainly imported by Canada, Japan and Europe. The US market is protected from Chinese imports by high anti-dumping duties, and the country's magnesium comes from Israel, or is domestic primary and secondary production.International trade in raw magnesium fell from about 500 thousand tons in 2007 to 305 thousand tons in 2009, according to Global Trade Atlas analyzed by Roskill. , grew to 480 thousand tons in 2011, but fell slightly in 2012.
About 50 thousand tons of waste and scrap were sold in 2012 (compared to 62 thousand tons in 2007), mainly exports from Canada, Germany and Austria and imports to the USA, Czech Republic and Hungary. In addition, approximately 110 thousand tons were sold in 2012 in the form of sawdust, shavings, granules and powder, with mainly exports from China and imports to Germany, Turkey and Canada. Finally, 37 thousand tons of forged products were sold in 2012 (compared to 46 thousand tons in 2011), and these were mainly exports from China, Austria and Germany, and imports to Taiwan, New Zealand and the UK.
Magnesium demand
Global apparent consumption (production + import - export) of magnesium reached 1050 thousand tons in 2007, an average annual growth rate of 8% compared to 630 thousand tons consumed in 2001. Consumption of primary magnesium metal fell by 7% in 2008 and a further 15% in 2009, falling below 690 thousand tons, as the global economic crisis led to a significant decline in demand for magnesium-containing products.
However, the market recovered, exceeding 2007 levels in 2011 and showing a new peak in demand in 2012. Recycling of magnesium has further increased consumption, with total magnesium consumption exceeding 1 million tons in 2007 and 1.1 million tons in 2012.
China dominates global consumption with 340 thousand tons in 2012, 33% of the total. Other major markets for magnesium are North America (23% of global consumption) and Europe (18%). Russia and Japan are also large consumers, together accounting for 12%.
Historically, aluminum alloys have been the primary application of magnesium worldwide, although in 2012, magnesium consumption in this end use and magnesium consumption in die casting alloys were equal, with each application accounting for approximately 365 k. tons, or 33% of total consumption. The packaging industry is the largest market for magnesium in aluminum alloys, followed by transportation, construction and consumer durables.
The automotive industry is by far the largest consumer of cast magnesium components. Magnesium alloy die casting is used for bodies, assemblies, brackets and other components for all layers of motor vehicles. The average magnesium use per vehicle in 2012 was 2.3 kg, with some models reaching 26 kg. Magnesium is used in the manufacture of die-cast housings for communication devices (such as mobile phones and smartphones), laptops, tablet computers and other electronic equipment. This is the second largest use of cast magnesium, after automobiles.
Titanium sponge (i.e., raw titanium metal) production was the third largest use of magnesium, accounting for approximately 123 kt or 11% of total global consumption in 2012, and desulfurization became the fourth largest use, with volume 119 thousand tons in 2012. The use of magnesium in steelmaking has decreased in recent years, due to the global economic crisis and the resulting slowdown (or decline) in steel production in many countries. On average, approximately 50 g/t steel is used worldwide.
Source: Magnesium Metal: Global Industrial Markets and Prospects 2012, Roskill Information Services Ltd.
Magnesium is also used in other applications, for example, as a spheroidizing modifier for cast iron and as cathodic protection, a method of preventing corrosion by forcing all surfaces of a metal structure to be cathodes through the provision of external active metal anodes. Roskill estimates that magnesium use for these two applications was around 65 kt and 60 kt in 2012.
While rising vehicle production in some regions has boosted consumption since the 2008/09 slump, the market has been somewhat held back by lower European vehicle shipments. However, as a result of pressure from reducing emissions, growth in the use of magnesium in the transportation sector continues to outpace the use of the metal in traditional materials such as steel, and the injection molding market is forecast to grow 6-7% per year until 2022 . In aluminum alloys, magnesium is used primarily in packaging, and this market continues to show strong expansion due to economic growth in developing countries.
Lighter vehicle weights and China boost magnesium demand
Roskill estimates that magnesium consumption reached a new peak in 2012 at 1.1 million tonnes, with demand increasing by 5.5% per year over the last decade. The largest magnesium consuming industries remain the die casting industry and aluminum alloys, each accounting for a third of total consumption. The transportation industry is the largest consumer of castings and the second largest consumer of the metal, after packaged aluminum-magnesium alloys.
The magnesium industry is benefiting from growth in automotive production, led by China, as well as rising vehicle magnesium consumption as manufacturers strive to meet government emissions reduction targets and rising fuel costs impact consumer purchasing trends. Continued weight loss efforts mean that magnesium intake will continue to rise by at least 5.0% per year until 2022. The use of magnesium in cast parts is likely to grow faster, at 6.5% per year, but the market will be held back by lower growth rates in steel desulfurization and spheroidizing annealing.
Growth in Chinese consumption has more than offset the slight decline in the rest of the world since 2007, and Asia accounted for 43% of the global total in 2012, up from 35% five years ago. North America accounted for 20% of consumption and Europe 15%. India and South Korea have shown strong consumption growth over the past five years, but from a low base in volume terms, while consumption in Russia has almost doubled due to increased titanium production. Asia, more specifically China, will continue to show the fastest growth in magnesium demand on a regional basis until 2022.
China dominates global supplies, but domestic competition is often overlooked
Primary magnesium production continues to be dominated by China, which Roskill estimates accounted for 75% of global production in 2012. Russia and the US together represent another 16%, followed by smaller producers Israel, Kazakhstan, Brazil, Serbia and Ukraine. Malaysia and South Korea have entered the market in recent years, albeit on a small scale, but that and some limited expansion of existing operations have done little to dilute China's growing share. Secondary magnesium, the production of which in 2012 amounted to 211 thousand tons, comes mainly from casting scrap. North America is the main source of recycled magnesium, followed by Europe, as these regions continue to be large consumers of magnesium-based products.
China's leading position in primary magnesium production reflects the domestic availability and low cost of ferrosilicon and energy (in the form of coal, coke and electricity), which are the main components of the energy-intensive, thermal pidging process of obtaining the metal. However, faced with rising energy prices and government pressure to reduce emissions, Chinese magnesium companies have invested in streamlining the process to reduce costs. Although China is often viewed as a single entity for magnesium supply, competition in the domestic industry has also increased greatly due to the recent increase in the availability of coke gas, as a result of domestic production shifting to Shaanxi province, which has limited growth in Shanxi and Ningxia provinces, and in resulting from production losses elsewhere.
Low capital costs in the transition from bench-scale plants mean that moving domestic production from province to province is relatively simple, but leads to significant capacity growth. Roskill estimates Chinese primary capacity at 1.3 million tons, but of this only 0.8-0.9 million tons are in use; the remaining capacities are mothballed or uneconomical. This trend led to the closure of at least one major manufacturer in China in 2012, as well as industry consolidation.
Despite price competitiveness and excess capacity in China, a new 100,000-ton electrolytic plant in Qinghai province, due to open soon, could further transform the domestic landscape. Several companies using new processes or variations of existing electrolytic and thermal methods also continue to explore the possibility of primary magnesium production in other countries, especially Australia and Canada. However, until these projects can compete with Chinese production costs and be economically viable at current and projected magnesium prices of $2,500-$3,000/t, China looks set to gradually increase its market share as demand increases.
Magnesium prices
There are no platforms for trading magnesium in the world and therefore, in most cases, contract terms are negotiated directly between producers and consumers. However, a large volume of Chinese material is sold on a spot basis by traders and Chinese manufacturers to the European, Japanese and domestic markets. The main market prices for magnesium are therefore Chinese domestic and export prices for the 99.8% Mg purity metal, and European ex-Rotterdam warehouse prices. Some magnesium supplies occur outside of China's trade with other countries, but they form a smaller part of the overall open market.
Increased demand, particularly in China, led to rapid price increases in the fourth quarter of 2007 and the first half of 2008. At their peak in the first half of 2008, prices rose above $6,000/t FOB China for 99.8% pure magnesium ingot. In subsequent years, prices retreated to lower levels, driven by reduced demand due to the global economic crisis, although they remained higher than before the 2007/08 peak. The removal of the 10% export duty on Chinese shipments at the end of 2012 caused a ripple effect on both European and Chinese export prices, driving prices to $2,500-$3,000/t FOB China from 2013. Due to anti-dumping duties on Chinese material, magnesium is sold at a premium in the US.
Source - Roskill.com, Ereport.ru
Other articles in the “Commodity Markets” section
Magnesium in organisms
Magnesium is found in all plants and animals. The substance is concentrated in some marine organisms. The maximum amount of metal element is contained in lime sponges - up to 4%.
This reagent is also part of the chlorophyll of green plants . In total, they account for 100 billion tons of the substance. Scientists have discovered magnesium in all components necessary for the cells of living organisms to exist. This element triggers many enzymes and allows plant chromosomes and colloidal systems to remain stable. In addition, the reagent maintains turgor pressure in cells. Thanks to magnesium, plants better absorb and assimilate phosphorus in the soil.
People and animals obtain the metal element through food. The daily intake for humans is from 0.3 to 0.5 g. In children and pregnant women, the need for the substance is slightly higher. A healthy person should have about 4.3 mg/% magnesium in their blood. In the body, the main consumer of the reagent is the liver, but most of the metal absorbed by it gradually passes into the bones and muscles.
Excess and deficiency
Magnesium is practically harmless to the body, although some of its compounds are assigned hazard class II. Basically, metal in different forms brings only benefits. And people, animals and plants most often suffer from a lack or excess of the substance.
When there is too little metal in the body, the risk of developing diabetes, kidney and intestinal diseases increases. People suffering from a lack of microelements often have headaches, insomnia, muscle spasms, and fatigue quickly sets in. If left untreated, this can lead to various more serious diseases, increasing the risk of cancer.
Before you begin to restore the level of a substance in the body, you must consult a doctor and determine the degree of need for the substance. After passing the tests, the doctor will prescribe a variable or constant intake of appropriate medications over a period of time, which should be taken strictly according to the instructions.
When there is an excess of microelement, people develop the following diseases:
- arthritis;
- speech disorder;
- nausea;
- drowsiness;
- and so on.
When the soil contains insufficient amounts of the substance, plants begin to develop leaf marbling and chlorosis. Lack of magnesium in the diet causes grass tetany in cattle.
Magnesium is one of the vital microelements. It is difficult to overestimate the role of this substance in animal and plant organisms. Its absence in sufficient quantities can cause many diseases.