The discoveries made by D.K. Chernov were published in 1868, due to which he gained worldwide fame and is deservedly considered the founder of metallography. Thanks to his discoveries, correct, scientifically based heat treatment of metals and metal alloys became possible.
The followers and students of D.K. Chernov - N.S. Kurnakov, A.A. Baykov and others - further contributed with their scientific works and research to the even greater development of domestic metallurgy.
2. Crystal structure of metals
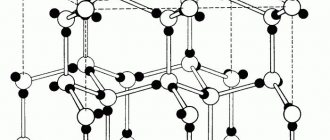
There are amorphous and crystalline bodies. The structure of amorphous bodies consists of randomly arranged atoms. Such bodies include, for example, glass, amber, resins, etc. Crystalline bodies differ from amorphous ones in that the atoms in them are arranged in a geometrically correct order. Metals and metal alloys are typical crystalline solids. Atoms, located in metals in a strictly defined geometric order, form a crystal lattice (Fig. 11). Depending on the arrangement of atoms, different types of crystal lattices are formed.
In metals, crystal lattices in the form of a centered cube, a face-centered cube, and a hexagonal prism are most often found.
For example, metals such as chromium, vanadium, tungsten, molybdenum and a number of others have a crystal lattice in the form of a centered cube (Fig. 12, a), in which eight atoms are located in the corners of the cube and one in the center of the cube.
Aluminum, copper, lead, nickel, silver, etc. have a crystal lattice - face-centered, that is, in the form of a cube with centered faces (Fig. 12.6). In such a lattice, there is one atom in each corner of the cube and one atom in the center of each face. There are therefore 14 atoms in total.
Metals such as, for example, zinc, titanium, and manganese have a crystal lattice in the form of a hexagonal prism (Fig. 12, e). The arrangement of atoms in a crystal lattice of the hexagonal prism type is as follows: there is one atom in each corner of the prism, one atom in the center of the upper base, one atom in the center of the lower base and three atoms in the middle section.
Rice. 11. Crystal lattice
Rice. 12. Types of crystal lattices: a - centered cube; b - face-centered cube; c - hexagonal prism
The distances between atoms in a crystal lattice are extremely small and are measured by a special unit of length called the angstrom (named after the scientist). One angstrom is equal to one hundred millionth of a centimeter.
In a molten liquid metal, atoms are in motion. Their movement is chaotic, but as the temperature of the metal decreases and approaches the critical temperature, i.e., the solidification temperature, so-called crystallization centers, or crystallization nuclei, are formed in it. Crystallization centers are extremely small groups of atoms that are grouped in a geometrically regular order.
The resulting crystallization nuclei are very unstable, and many of them dissolve again. Practical observations have established that crystallization nuclei acquire stability and begin to grow when the liquid metal is supercooled to a certain temperature. The cooling curve of pure metal gives a clear idea of how the crystallization process proceeds.
Rice. 13. Cooling curve of pure metal
In a supercooled metal (Fig. 13, a), the crystallization process begins to proceed faster. After the onset of intensive crystallization, the temperature of the supercooled metal rises to the temperature of its solidification (b) due to the release of latent heat of crystallization.
Rice. 14. Scheme of grain formation
During the entire crystallization process, the temperature of the metal remains constant (b, c). After the metal passes from the liquid to the solid state, its temperature begins to decrease to ambient temperature (g). During the crystallization process, nuclei grow due to atoms from the surrounding liquid, which are located in the crystal lattice in a strictly defined order (Fig. 14, a, b). Initially, the growth of crystallization nuclei occurs freely, and they have a regular external geometric shape. But since many nuclei are formed at the same time, a moment comes when they begin to meet each other (Fig. 14, c, d, e). After such a collision, their growth becomes possible only in those directions where there are no obstacles. This leads to the fact that the external geometric shape of the metal crystals becomes irregular, as a result of which they are usually called metal grains (Fig. 14, f).
3. Change in structure in solid metals (allotropy phenomenon)
The structure of some metals in the solid state can, at a certain temperature, undergo transformations, which represent a rearrangement of atoms and a transition from one type of crystal lattice to another. This phenomenon is called allotropy of metals. The different crystalline forms into which the same solid metal crystallizes at certain temperatures are called allotropes. Allotropic modifications are designated by Greek letters. The transition from one modification to another occurs at a certain, constant temperature and is accompanied by the absorption of heat (during heating) or the release of heat (during cooling) and the formation of a new crystal lattice.
Rice. 15. Cooling curve of pure iron
Pure iron exists in several modifications. The cooling curve of pure iron (Fig. 15) shows at what temperatures allotropic transformations of iron occur. Up to a temperature of 910°, iron has a crystal lattice in the form of a centered cube and is called alpha iron a-Fe. Moreover, up to 770° a-Fe is magnetic, and above 770° it is non-magnetic. At a temperature of 910°, the a-Fe crystal lattice changes and becomes face-centered; this modification is called gamma iron y-Fe and is stable up to a temperature of 1390°, at which it again turns into a centered cube lattice. The new modification is called delta iron 8-Fe. Allotropic transformations are very important, since metals undergoing such transformations can be subjected to heat treatment. In addition to iron, some other metals are also subject to allotropic transformations, such as titanium, manganese, cobalt, zirconium, and tin.
4. Structure of alloys
An alloy is a complex substance obtained by fusing two or more elements. The elements that make up an alloy are called alloy components. In the liquid state, an alloy is a solution in which the atoms of one component are evenly distributed between the atoms of other components, due to which the liquid solution has the same properties in any part, no matter how small it is. Such substances are called homogeneous. The properties of any liquid solution differ from the properties of its components, but each component influences the nature of the properties of the solution. Upon careful examination of liquid solutions, it turns out that the physical, electrical and other properties of these solutions differ sharply from the properties of their components and can vary depending on the percentage of components, i.e., on the concentration of the solution.
The concentration of a solution is the ratio of the weight of the soluble substance to the weight of the entire solution. Concentration is usually expressed as a percentage. When an alloy transitions from a liquid to a solid state, various types of interaction between components can occur. The main types of interaction of components are: mechanical mixture, chemical compound and solid solution.
A mechanical mixture represents a type of interaction of components in which, during crystallization, the components of the alloy do not enter into a chemical reaction and do not dissolve in one another, but retain their crystal lattices. Consequently, the structure of an alloy that is a mechanical mixture of any two components, for example, lead and antimony, will consist of extremely small lead crystals and antimony crystals.
In the case of a chemical compound, the interaction of the alloy components is characterized by the formation of a completely new crystal lattice, unlike the crystal lattices of the components; in this case, the ratio of components will always be strictly defined.
A solid solution differs from a mechanical mixture and a chemical compound in that it retains the crystal lattice of the solvent metal, in which the atoms of all the components of the alloy are located. A metal whose crystal lattice is retained after the formation of a solid solution is called a solvent. Solid solutions can be of two types: interstitial solid solution and substitutional solid solution. In an interstitial solid solution, the atoms of the solute are located between the atoms of the solvent (Fig. 16, a). In a solid solution of substitution, the atoms of the solute partially replace the atoms of the solvent in its crystal lattice (Fig. 16.6).
Rice. 16. Solid solution lattice: a - interstitial; b - substitutions
5. State diagram of alloys (lead - antimony) and its construction
To study alloys, phase diagrams of alloys are usually used. Alloy phase diagrams replace all records and alloy cooling curves obtained as a result of Numerous observations. Such a diagram makes it possible to see all changes in the structure of the alloy and its properties that occur depending on changes in concentration and temperature. Any point on the diagram characterizes a splase of a certain concentration and structure. From the phase diagram of alloys, you can determine the melting point and solidification temperature of a given alloy at any concentration. Knowledge of these facts contributes to the correct choice of heating and cooling temperatures during thermal and chemical-thermal processing of various alloys.
In order to understand how the state diagram of alloys is constructed, let us consider the construction of such a diagram for alloys of lead and antimony. Let's take the pure metals lead and antimony and several of their alloys with antimony contents of 5%, 10%, 13%, 20%, 40% and 80%.
Rice. 17. Cooling curves of lead, antimony and various lead-antimony alloys
To determine the critical points of the selected metals and their alloys, we heat each metal and alloy in turn until complete melting and, using a thermocouple or pyrometer, carefully monitor the cooling process and construct cooling curves (Fig. 17). During the cooling process of molten pure lead, the following phenomena will occur.
At temperatures above 327°, lead is in a liquid state (Fig. 17, a); at a temperature of 327°, the process of crystallization of lead is observed with a delay in the temperature drop until crystallization is complete; After crystallization is completed, solid lead is further cooled to ambient temperature.
Similar phenomena are observed during the cooling process of molten pure antimony (Fig. 17, b), with the only difference being that the crystallization of antimony begins at a temperature of 630°.
An alloy consisting of 95% lead and 5% antimony (Fig. 17, c) has a cooling curve with two critical points, so it solidifies in the temperature range 296-246 °. At a temperature of 296°, the first crystals of pure lead begin to separate from the liquid alloy. The curve at this point has an inflection. As the temperature decreases further, the number of lead crystals will increasingly increase, and the remaining part of the liquid alloy will be enriched in antimony. This phenomenon continues until the concentration of the liquid alloy reaches 13% antimony and 87% lead; at this concentration, the entire alloy that remains liquid will solidify at a temperature of 246°. An alloy consisting of 30% lead and 10% antimony (Fig. 17, d) will also harden in the temperature range 260-246°. At a temperature of 260°, lead crystals begin to separate from the liquid alloy. When the concentration of the liquid alloy reaches 13% antimony and 87% lead, the alloy solidifies at a temperature of 246° (Fig. 17, d). Consequently, when the above alloys are cooled, before the critical temperature of 246° is reached, all excess lead in excess of 87% is released from the liquid alloy in the form of crystals. Upon reaching the composition of 87% lead and 13% antimony, the alloy becomes solid at a temperature of 246°. The structure of a solidified alloy of this concentration consists of regularly alternating particles of lead and antimony. Such a mechanical mixture is called eutectic. All lead-antimony alloys containing less than 13% antimony will always have an excess of lead and, upon cooling, tend to release this excess in the form of solid lead crystals in order to form a eutectic at a temperature of 246°. Then, obviously, in the solid state such alloys will have a lead + eutectic structure. An alloy consisting of 87% lead and 13% antimony has a cooling curve (Fig. 17, e) with one critical point. This alloy is in a liquid state at temperatures above 246°. At a temperature of 246° the alloy completely transforms into a solid state.
This structure of the hard alloy is a pure eutectic. An alloy consisting of 80% lead and 20% antimony (Fig. 17, e) is in a liquid state at temperatures above 280°. When the alloy is cooled to a temperature of 280°, crystals of solid antimony begin to separate from it, and this process will continue until the remaining liquid alloy takes on a eutectic composition. At a temperature of 246° the entire alloy solidifies. The structure of the solidified alloy will consist of antimony and eutectic crystals. An alloy consisting of 60°/0 lead and 40°/0 antimony (Fig. 17, g), above a temperature of 395°, is in a liquid state. At a temperature of 395°, the crystallization process begins with the release of excess antimony crystals from the liquid solution. Upon reaching the eutectic composition (87o/0 lead and 13% antimony) at a temperature of 246°, the entire alloy passes into the solid state, forming a structure consisting of crystals of antimony and eutectic.
An alloy consisting of 20% lead and 80% antimony (Fig. 17, h) is in a liquid state above a temperature of 570°. At a temperature of 570°, the process of separation of excess antimony crystals from the liquid alloy begins. Upon reaching the eutectic composition at a temperature of 246°, the entire alloy transforms into a solid state. The alloy structure consists of antimony and eutectic crystals. The above observations show that all lead-antimony alloys, in which the lead content is less than 87o/0, contain an excess of antimony and, upon cooling, will tend to release this excess during crystallization in the form of solid antimony crystals in order to form a eutectic at a temperature of 246°. The more antimony there is in the alloy, the higher the temperature, the excess (versus 13<>/0) antimony will begin to separate from it during cooling. Alloys of lead with antimony, if they contain excess antimony, form a structure in the solid state consisting of crystals of antimony and eutectic.
Rice. 18. State diagram of alloys of the lead-antimony system
The cooling curves of lead and antimony alloys with different percentages of Components can be combined into one state diagram for lead-antimony alloys. To do this, let us plot the content of lead and antimony in the tested alloys on the horizontal axis (Fig. 18). Through the points corresponding to 100% antimony and 100% lead, we draw vertical straight lines, on which we plot temperatures from 0 to 700°. We draw dotted vertical lines through the points corresponding to the compositions of the tested alloys. After this, we transfer the critical points from the cooling curves to the vertical lines of the diagram. We denote the critical point of pure lead (327°) by the letter A, and the critical point of pure antimony (630°) by the letter C. As is known from previous observations, each alloy has two critical points, except for the eutectic alloy. Let us denote the critical temperature of the eutectic alloy by the letter B. Let us connect points A and C with smooth curves to point B so that the curves pass through all the upper critical points. We draw a straight line through all the lower critical points, which will also pass through point B, and denote its left end with the letter D, and the right end with the letter E. The upper critical points are the starting points of the solidification of the alloys, and the lower critical points are the end points of the solidification of the alloys. The ABC line of a diagram is called the liquidus line (from the Latin word liquid). Above line ABC, all lead and antimony alloys are in a liquid state. The DBE line is called the solidus line (from the Latin word for "solid"). Below the DBE line, all alloys of lead with antimony are in the solid state, and below the DB line they will consist of crystals of lead and eutectic and are called hypoeutectic, below point B - from pure eutectic (the so-called eutectic) and below the line BE - from crystals of antimony and eutectics (hypereutectic).
6. Structural components of iron-carbon alloys
There are various structural components of iron-carbon alloys. They have the following names: ferrite, cementite, austenite.
Ferrite is the name given to chemically pure iron, as well as a solid solution of carbon in iron. The solubility of carbon in iron is extremely low and is usually 0.006-0.04%. Ferrite is stable up to a temperature of 910°. It has low hardness and low strength. The hardness of ferrite depends on the grain size; The plasticity of ferrite is high.
Cementite is a chemical compound of iron and carbon. Cementite contains 6.67% carbon (by weight) and is a very hard and brittle crystalline substance that, when heated to high temperatures, breaks down into ferrite and free carbon (annealing carbon). White cast iron contains a large amount of cementite. Cementite has a significant effect on the mechanical properties of steel.
The mechanical mixture of ferrite and cementite forms the steel structure called pearlite. Perlite comes in two types: lamellar, or striped, and granular. Lamellar pearlite has the appearance of alternating very small plates of ferrite and cementite. By heating to certain temperatures, it is possible to change the structure of lamellar pearlite and obtain the so-called granular pearlite, in which cementite is in the form of round grains located among ferrite.
Granular perlite has better mechanical properties than lamellar perlite. In terms of its mechanical properties, pearlite occupies an intermediate position between ferrite and cementite. Steel with a carbon content of 0.83% has a pure pearlite structure.
Austenite is an interstitial solid solution of carbon in iron. The solubility of carbon in y-iron can reach 1.7%. In ordinary carbon steel, austenite is stable up to a temperature of 723°. Below 723° it decomposes into ferrite and cementite. At temperatures below 723°, austenite can only be preserved in high-alloy manganese, chromium-nickel or nickel steels.
A eutectic mixture of austenite and cementite forms a steel structure called ledeburite. Ledeburite is formed when an iron-carbon alloy with a carbon content of 4.3% solidifies at a temperature of 1130°. Ledeburite remains stable up to a temperature of 723°. Below this temperature, ledeburite changes its structure, since the austenite included in its composition disintegrates into pearlite, as a result of which ledeburite at temperatures below 723° will consist of pearlite and cementite.
7. State diagram of iron-carbon alloys
Steels and cast irons are complex alloys containing, in addition to iron and carbon, other elements - silicon, manganese, phosphorus and sulfur, as well as non-ferrous metals (in alloy steels and cast irons). The main component that determines the nature and properties of an iron-carbon alloy is carbon. The structure and properties of steel and cast iron change only if they are heated to critical temperatures, depending on the carbon content in these alloys. The critical temperatures of iron-carbon alloys with different carbon contents can be plotted on a special diagram called the state diagram of alloys of the iron-carbon system.
Such a diagram (Fig. 19) makes it possible to determine for each alloy of steel and cast iron its melting point, all transformations experienced by the alloy during cooling and heating, and the structure of the alloy at any temperature. The horizontal axis of the diagram represents carbon content as a percentage, and the vertical axis represents temperature. Each point on the diagram represents a specific alloy at a specific temperature. Above the ACD line, all alloys are in a liquid state. The ACD line is the liquidus line.
Pure iron melts and solidifies at one point at a temperature of 1535°. All other alloys of iron and carbon melt and solidify in a certain temperature range that gradually changes. Alloys containing from 0 to 4.39% carbon begin to harden along the AC line, releasing solid austenite crystals. Alloys containing more than 4.3% carbon begin to harden along the CD line, releasing solid Fe3C cementite crystals. An alloy containing 4.3% carbon solidifies completely at point C, releasing both austenite and cementite crystals, resulting in the formation of a eutectic called ledeburite. The AECF line is the solidus line. Below this line all alloys are in a solid state. The area of the diagram limited by the lines AC, CE, EA represents alloys consisting of solid crystals of austenite and a liquid alloy; area of the diagram bounded by DC lines. CF, FD, includes alloys consisting of solid cementite crystals and a liquid alloy.
Rice. 19. State diagram of the iron-carbon system
Alloys located in the area of the diagram limited by lines AE, ES, SG consist of austenite. Along the ES line, cementite begins to precipitate from austenite. Below the PSK line, all remaining austenite decomposes at point 5 into ferrite and cementite, forming a mechanical mixture called pearlite, and at point 5 the alloy contains 0.83°/about carbon. Such an alloy is called eutectoid. The GPQ line shows the saturation limit of a-iron with carbon.
Steels with a carbon content from 0 to 0.83% are called pre-eutectoid, steels with a carbon content of 0.83°/o are called eutectoid, and steels with a carbon content from 0.83 to 1.7% are hypereutectoid.
Cast iron with a carbon content of 1.7 to 4.3% is called hypoeutectic cast iron, cast iron with a carbon content of 4.3% is called eutectic, and with a carbon content of 4.3 to 6.67% is hypereutectic.
8. Change in steel structure
When steel is heated above the critical point Aci (Fig. 20) (the temperature at which pearlite transforms into austenite), transformations, as is known, begin to occur in the structure of the steel. After the transformation is completed, further heating or holding leads to the growth of austenite grains. Grain growth occurs spontaneously, and the rate of this process increases with increasing temperature.
The growth of austenite grains occurs in different ways and depends on the tendency of the grain to grow. Depending on this, a distinction is made between hereditarily coarse-grained and hereditarily fine-grained steels. Heredity refers to the tendency of grain to grow. Hereditarily coarse-grained steels have an increased tendency of austenitic grains to grow, and hereditarily fine-grained steels have a low tendency to grow.
The change in grain size upon heating of these steels can be seen from Fig. 20. When steel is heated above the critical point Acx, the grain size of the steel sharply decreases. With further heating, the austenitic grain in hereditarily fine-grained steels does not grow until temperatures of the order of 950-1000°, after which rapid grain growth begins.
In hereditarily coarse-grained steels, the grain begins to grow immediately after passing through the critical point Ac\. The size of the austenite grain is of great importance for obtaining the final results when heat treating steels. The transformation of pearlite and austenite is accompanied by grain refinement. The grain formed during this transformation is very fine. During the reverse transformation of austenite grains into pearlite grains, almost no changes in its size occur (Fig. 21). Therefore, the pearlite grain size depends mainly on the austenite grain size. And since the austenite grain grows only when heated, then by heating the steel to certain temperatures, the final required grain size of the steel can be obtained. The size of the actual grain of steel, i.e. the grain obtained as a result of one or another heat treatment, has a great influence on the mechanical properties of steel.
Rice. 20. Scheme of grain growth in hereditarily fine-grained and hereditarily coarse-grained steel
Coarse-grained steel is easily calcined and processed by cutting tools, but at the same time it is more prone to hardening deformations and the formation of cracks in it. Fine-grained steels have greater impact strength compared to coarse-grained steels, but less hardenability. Fine-grain steel is used to make products that require a tough core on a hard surface.
When heated steel is slowly cooled to the austenitic state, austenite transforms into pearlite, ferrite and cementite. At high cooling rates - from 40 to 200° per second or more - as a result of the decomposition of austenite, steel structures are obtained: sorbitol, troostite and martensite.
There are two types of sorbitol: hardening sorbitol and tempering sorbitol. Hardening sorbitol consists of alternating plates of ferrite and cementite, but the cementite plates in it are much thinner than in pearlite. Sorbitol is harder than perlite, but has less viscosity. Tempered sorbitol is obtained as a result of the decomposition of martensite in steel when it is tempered in the temperature range 500-600°. In tempered sorbitol, the cementite particles are spherical in shape. Troostite, like sorbitol, is of two types: hardened troostite and tempered troostite. Troostite is a mechanical mixture of ferrite and cementite plates, but thinner than in sorbitol. Troostite has greater hardness compared to sorbitol, but lower viscosity. Tempered troostite is a product of martensite decomposition when tempered in the temperature range 350-450°. Martensite is an interstitial solid solution of carbon. Martensite crystals are needle-shaped. It has high hardness and good wear resistance; its plasticity and viscosity are low.
Rice. 21. Change in grain size during recrystallization
Types of alloys
According to the method of manufacturing alloys, a distinction is made between cast and powder alloys. Cast alloys are produced by melt crystallization of mixed components. Powder - by pressing a mixture of powders followed by sintering at high temperature. The components of a powder alloy can be not only powders of simple substances, but also powders of chemical compounds. For example, the main components of hard alloys are tungsten or titanium carbides.
According to the method of obtaining the workpiece (product), a distinction is made between casting (for example, cast iron, silumin), wrought alloys (for example, steel) and powder alloys.
In a solid aggregate state, an alloy can be homogeneous (uniform, single-phase - consists of crystallites of the same type) and heterogeneous (inhomogeneous, multiphase). The solid solution is the basis of the alloy (matrix phase). The phase composition of a heterogeneous alloy depends on its chemical composition.
The alloy may contain interstitial solid solutions, substitutional solid solutions, chemical compounds (including carbides, nitrides, intermetallic compounds) and crystallites of simple substances.
Properties of metals
Characteristic properties of metals
Metallic luster (characteristic not only of metals: non-metals iodine and carbon in the form of graphite also have it)
Good electrical conductivity
Can be easily machined (see: ductility; however, some metals, such as germanium and bismuth, are not ductile)
High density (usually metals are heavier than non-metals)
High melting point (exceptions: mercury, gallium and alkali metals)
· Great thermal conductivity
· They are most often reducing agents in reactions
Types of alloys
According to the method of manufacturing alloys, a distinction is made between cast and powder alloys. Cast alloys are produced by melt crystallization of mixed components. Powder - by pressing a mixture of powders followed by sintering at high temperature. The components of a powder alloy can be not only powders of simple substances, but also powders of chemical compounds. For example, the main components of hard alloys are tungsten or titanium carbides.
According to the method of obtaining the workpiece (product), a distinction is made between casting (for example, cast iron, silumin), wrought alloys (for example, steel) and powder alloys.
In a solid aggregate state, an alloy can be homogeneous (uniform, single-phase - consists of crystallites of the same type) and heterogeneous (inhomogeneous, multiphase). The solid solution is the basis of the alloy (matrix phase). The phase composition of a heterogeneous alloy depends on its chemical composition.
The alloy may contain interstitial solid solutions, substitutional solid solutions, chemical compounds (including carbides, nitrides, intermetallic compounds) and crystallites of simple substances.
Test on the topic
- /10
Question 1 of 10What explains the reducing properties of metals?
Start test
Hall of Fame
To get here, take the test.
- Galina Skotynyanskaya
7/10
- Alexander Kotkov
10/10
- Hoyr Hftx
10/10
- Alexander Kotkov
10/10
- Sergey Efremov
6/10