In this article:
1. Methods for direct reduction of iron, characteristics of products and raw materials
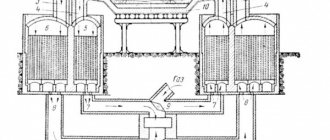
2. Iron production in shaft furnaces (Midrex technology)
3. Iron production in periodically operating retorts (HYL/Energiron technology)
4. Iron production on a moving grate
5. Iron production in rotary tube furnaces
6. Iron production in fluidized bed reactors
7. FASTMET and ITmk3 units
8. Chemical-thermal method of obtaining iron
Iron production
Iron production is based on the carbothermic reduction of oxide metal-containing ores. Sulfide, arsenide and other ores are preliminarily subjected to oxidative roasting. The possibility of using coal (coke) and its oxide as a reducing agent is due to the fact that iron is a metal of medium activity.
Iron and steel production: Coal is heated in furnaces without air to remove gas (volatile material) and produce coke. Coke is used in a blast furnace to smelt metal. Coal can also be directly introduced into the blast furnace by adding pulverized coal.
Iron production in our country has been known since prehistoric times. Archaeological excavations of ancient settlements in the central part of the USSR, the Urals, Ukraine, Belarus, Transcaucasia and a number of other areas show that our distant ancestors already 2-5 - 3 millennia ago were able to obtain iron from ores and make weapons and tools from it and household items. For many centuries there has been a cheese-making method for obtaining iron.
The production of iron, cast iron and steel, or the so-called ferrous metallurgy, is the basis of the country's industrialization. The Communist Party and the Soviet government have attached and continue to attach great importance to the development of ferrous metallurgy.
The production of iron (more precisely steel) is carried out in two stages: a) first, cast iron is smelted from iron ores, part of which is used for various products; b) the rest of the cast iron is processed into steel.
The production of iron (more precisely steel) is carried out in two stages: a) first, cast iron is smelted from iron ores, part of which is used for various products; b) the rest of the cast iron is processed into steel.
The production of iron from ores is based on the reaction of its reduction from oxygen ores with carbon (charcoal or coke), as well as carbon monoxide. But during the production process, molten iron dissolves carbon in itself, as a result of which not pure iron is obtained, but an alloy of it with carbon and other impurities, called cast iron.
Iron production on a large scale is very economical; iron has a number of useful mechanical properties, especially in alloys with other elements. Steel, an essential structural material, consists primarily of iron containing small amounts of carbon and some other elements.
Iron production, brought to colossal proportions by the railway fever of 1845, suffered, of course, to the extent that sales for the excessive amount of iron produced were reduced. In the cotton industry, the main industry for the East Indian and Chinese markets, there was already an overproduction of goods produced for that market as early as 1845, and a certain decline very soon set in. A poor cotton harvest in 1846, rising prices of both raw materials and finished goods, and the resulting decline in consumption, added to the depressed state of this industry. In the early months of 1847, production fell significantly throughout Lancashire, and the workers of the cotton industry were already under the blow of the crisis.
The production of iron, cast iron and steel, or the so-called ferrous metallurgy, is the basis of the country's industrialization. The Communist Party and the Soviet government have attached and continue to attach great importance to the development of ferrous metallurgy.
Methods of iron and steel production in which the thermal energy of a nuclear reactor is used to produce hot reducing gas, which is used to reduce oxidized iron ores.
Unsurpassed in casting
The blast furnace process produces three types of cast iron:
- foundry (gray),
- conversion (white),
- special cast iron (ferroalloys).
Casting from gray cast iron works well in compression, is two times weaker in bending and three to four times weaker in tension - these properties must be taken into account when designing art products intended for casting. Gray cast iron, due to its high corrosion resistance, is extremely widely used in the manufacture of exterior products: park decorative sculptures, vases and fountains, garden fences, gates, tombstones and gratings. And its durability and abrasion resistance make it an indispensable material for steps and stair railings.
Finally, exceptionally high casting properties make it possible to cast the finest openwork objects with a beautiful black-brown color. Small household items are also excellently made from cast iron: ashtrays, door handles, toiletries and even watch chains.
Bulat (steel): where did it come from and who used it
The first information about damask steel came 2,300 years ago from participants in the famous campaign of Alexander the Great to India.
The warriors said that Indian blades cut stones and cut light fabrics in the air.
Perhaps it was this information that Walter Scott used in his novel “The Talisman”.
It describes a contest of agility between Sultan Saladin and the English king Richard the Lionheart. Richard cut the spear of one of the knights into two pieces with his steel sword. In response, Saladin threw a blanket of the finest fabric into the air and cut it with his damask blade.
Damask steel actually first appeared in India.
The Indians sold wootz - “bread” made of steel - to the countries of the East. They were flat cakes with a diameter of 12.5 cm and a thickness of 0.25 cm. The woots weighed about 900 grams. Such a “bread” was cut in half, into equal parts, so that the buyer could examine the structure of the metal.
Indian craftsmen mastered the art of steel processing to perfection.
“There will never be a people who would be better versed in certain types of swords and their names than the inhabitants of India,” wrote Biruni, who saw with his own eyes the production of steel and swords. He was especially struck by the colored swords. The Indians rubbed polished iron with hot copper sulfate powder, after which they obtained swords of various colors - green, blue, white and with patterns.
Among the many Indian swords, Biruni was most deeply impressed by a sword called “Majli”, on which animals and trees were depicted. Its cost was equal to the price of the best elephant. But if human figures were depicted on the sword, such weapons were even more expensive.
Patterns and drawings on metal were the main distinguishing feature of damask swords.
On some damask steel the patterns were visible to the naked eye immediately after polishing. On others they appeared only after etching with plant juice.
The pattern could be large or small.
Another place where excellent damask steel was produced was the city of Damascus. In the Middle Ages, swords came from Damascus to different countries. They could even be seen in African tribes. Damascus steel later became known as Damascus steel.
How people of the Middle Ages managed to create damask blades from stainless steel, which was unusually strong, was a mystery.
Various scientists in many countries tried to unravel the mystery of damask steel. The famous English physicist Mikhail Faraday tried to obtain damask steel by adding aluminum and platinum to steel.
In the end, the secret of damask steel was revealed by the Ural metallurgist Pavel Anosov. After many years of searching, trial and error, in 1837 he managed to make a damask blade in the city of Zlatoust. Anosov knew that damask steel production still existed in Moscow in the 16th-17th centuries.
He was familiar with the documents of that time, where there were entries: “Saber strip, blue damask steel, Moscow forged,” “Russian saber strip with fullers for damask steel.” By the end of the 17th century, the art of making damask steel fell into decline and was gradually forgotten. And now, more than two hundred years later, damask steel appeared in Zlatoust. “The strip of damask steel bent without the slightest damage and produced a clear and high ringing sound. The polished end crushed the best English chisels, while the loosened end easily accepted impressions and cut off cleanly and evenly,” Anosov wrote in the Mining Journal.
The damask blade prepared in Zlatoust had a golden tint and a large mesh or cranked pattern.
Experts believed that such a pattern was a sign of the highest grade of damask steel. The blade, made at the Zlatoust factory, cut through nails and bones without damaging the blade. With the help of these blades it was possible to perform the same trick with a thin blanket of gas that Saladin struck King Richard with.
People struggled with the riddle of damask steel for so long that they were extremely surprised when Anosov reported that damask steel is “iron and carbon and nothing more; it’s all about the purity of the starting materials, the cooling method, the crystallization.”
Bulat turned out to be a high-carbon steel without any special impurities, being a product of natural crystallization of steel obtained by combining iron and carbon.
The essence of the formation of damask steel was the saturation of the alloy with a large amount of carbon (about 1.3-1.5%). During slow cooling, a compound of iron and carbon was formed and found in some excess - the so-called cementite, which did not dissolve, as happens in ordinary steel, but remained in the iron as if in suspension. Layers of cementite were enveloped in slowly cooling soft iron.
Therefore, with a high carbon content, which gives the metal hardness, damask steel retains high flexibility and elasticity, which is not characteristic of ordinary steel. Due to the presence of layers of brittle cementite, forging damask steel must be done extremely carefully, with blows of a light hammer, with repeated heating to a critical temperature, that is, to a red-hot temperature. If it is raised higher, damask steel will lose its basic properties and its characteristic pattern. The process of making damask steel is labor-intensive, time-consuming and requires high skill.
While developing the process for producing damask steel, Anosov simultaneously invented a new method for producing steel by fusing unusable iron and steel scraps in clay pots, that is, crucibles, using high temperature air furnaces.
Having established the production of crucible steel in the Urals, Anosov reported that it was in no way inferior to English cast steel.
Nowadays, damask steel is not produced. The fact is that it was a product of handicraft production, and in general had only one use - for the manufacture of edged weapons. But modern technology has found many ways to produce steel of a wide variety of grades with various properties that damask steel did not possess.
Modern technology needs metals and alloys to operate at pressures of hundreds and thousands of atmospheres and in deep vacuum, when the pressure is close to zero. Cold-resistant steels must maintain strength at temperatures close to absolute zero (-273°C). Nuclear reactors require a metal with the highest magnetic conductivity; jet and rocket engines require steel that can maintain strength at very high temperatures and heavy loads.
The first mention of steel goes back to the distant 8-12 centuries BC. Even then, the troops of the Indian king Porus had strong and sharp weapons.
Indian craftsmen managed to obtain high-carbon steel, called damask steel. Its production was difficult and the production secret remained undisclosed.
Multi-colored metal with a pattern
It is not unusual for any of the metals known to us to change color when subjected to any kind of processing. The “palette” of a particular metal depends on the degree of heating, the processing itself, and the chemical properties. But it is impossible to imagine blue gold or red silver. On the contrary, iron, and accordingly steel and cast iron in all their “guises,” has a color range incomparable to any other metal.
When cold, it can be gray, black, almost white, blue, and blue, golden and reddish. Moreover, iron is the only metal that can decorate itself with decorative patterns that appear as if from within. The variations of this textured ornament are endless, and they cannot be classified as one of the generally known ones, since this pattern is created by the metal itself.
History of iron production and use
Steel is an alloy of iron and carbon.
Thanks to carbon, steel becomes hard and durable, while the toughness and ductility of iron decreases.
Carbon percentage up to 2.14.
In ancient times, people found metals in nature. At first they were just decoration.
Then copper tips for spears and arrows appeared. Iron was worth its weight in gold until man learned to smelt it from ore in furnaces, marking the beginning of the Iron Age.
Many years later, they managed to produce stainless steel and rolled metal, the cost of which you can find out by clicking on the link https://www.allmetal.ru/ .
Even ancient metallurgists noticed that the properties of a metal depend on its composition and its processing.
Then it was noticed that if iron was heated red-hot and then cooled in water, the hardness of the metal increased.
This type of hardening is still used in steel processing. Then each master had his own secret of hardening steel, but there was no explanation for why the metal became stronger.
Ancient alchemists tried to describe the process of metallurgy in theory. In the 13th century AD. the alchemist Magnus contributed by recording the transformation of iron into steel by distillation of the watery part and hardening.
He argued that steel becomes whiter due to the separation of impurities, and also noted that metal that is too strong eventually crumbles under the hammer.
Scientists of the following centuries continued to search for clues to the phenomena occurring in metal.
In particular, a book was published in Germany that described the properties of steel that make it indispensable for cutting tools and implements. It was noticed that when heated up and cooled slowly, the steel became soft. And with rapid cooling in liquid, the metal became extremely hard and lost its brittleness. The British for a long time kept the secret of hardening steel in molten lead or tin.
The history of steel production is the history of experiments on metals, an understanding of the transformation of iron.
Scientists have long unraveled the mystery of turning iron into a durable alloy. Numerous experiments yielded either a strong but brittle metal or a soft, bendable and quickly dull metal. It took the Russian scientist P.P. Anosov 10 years. to justify the production of durable, high-quality steel. Through trial and error, Anosov tried to uncover the secret of damask steel.
The successor of his ideas was D.K. Chernov, who described the transformation of ore into steel from a scientific point of view.
He managed to cast a block of high-quality steel and make damask daggers from it, and described the process in a scientific work. His important discovery was the discovery of the critical points of steel.
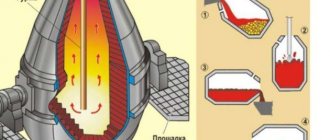
Nowadays, iron ore is smelted in huge blast furnaces at metallurgical plants.
The ore is first converted into pig iron. It is then melted in open hearths, turning into steel. This process is monitored by qualified specialists.
History of steel production
BC. Wrought iron was already being produced everywhere in Europe. Many magnificent Greek and Roman buildings were built of stone using butterfly-shaped iron tools covered with lead. In 500 BC. e. The Etruscans who lived on the west coast of Italy produced more than 4.5 thousand kilograms of iron per year.
Iron was forged in a forge, and charcoal was used to keep the fire going. The fire was fanned using special bellows made from animal skins. Later, the small stone furnaces were dismantled and mass iron smelting began. Ore was delivered to the furnaces on sailing ships. Due to the fact that the ore processing method used by the Etruscans was ineffective, its reserves were quickly depleted. In addition, charcoal production has sharply reduced the number of forests in western Italy.
The first steel was created by the Celts around 200 AD. e. They cut the wrought iron into thin strips and placed them in a container with burnt bones and charcoal, after which they heated the whole thing in a furnace for 10-12 hours at very high heat. As a result, the metal surface was enriched with carbon. Then they welded these strips together through forging and thus created knives. These knives became the predecessors of the blades that we mistakenly call Damascus.
The Celtic process for making steel in 1050 was copied by the Vikings and Germans. Since then, steel blades have been produced in these countries, the manufacturing method of which was strictly classified. Damascus steel was produced in Pakistan and sent in the form of damask steel to Syria, where the famous Damascus blades were made. The process of producing Damascus steel is very complex because it had to be heated to a very high temperature, and if the temperature was exceeded, the material could break.
Over time, the melting temperature of iron in furnaces became higher and higher, so the resulting iron contained 3-4% carbon.
It was fragile and suitable only for casting. It was impossible to make knives and parts for transport from it. In addition, by this time a huge part of the forests in Europe had been cut down for construction purposes and the production of charcoal.
Then the King of England issued a decree that no more forests could be cut down, and steel producers had to come up with a way to process coal into coke. In England, they developed a method for tinning steel by mixing molten iron with iron silicate and iron oxide. Iron silicate is one of the components of wrought iron.
Coal-fired furnaces were called the furnace. One worker had to stir the resulting mixture, which created carbon dioxide, so the melting point of the iron became higher, and the tinning process began.
Large pieces weighing from 90 kg to 130 kg were placed inside. Another worker, using a pair of large tongs, took these pieces and placed them under a press to squeeze out the iron silicate from them. After the press, the pieces were placed in a rolling mill, where they were formed into strips of cast iron.
These strips were cut into short pieces and joined together, after which they were placed in a recess filled with carbon and heated to welding temperature. After this, the strips of red iron were again sent to the rolling mill and graded iron was obtained. This method was used not only in Europe, but in the eastern United States.
To make steel, thin rolled products were placed in a cavity filled with carbon obtained from burning bones and heated at high temperatures for several days.
The carbon was absorbed by the iron, resulting in bubbly steel. Cement steel or tomlenka was called bubbly. This concept arose from the appearance of the strips recovered from the carbon pit, which were covered in bubbles. After this, the strips were folded together and forged, then folded again and forged, in this way high-quality steel was obtained.
England needed high-quality steel to create a fleet that could cross the ocean.
One enterprising Englishman noticed that glassblowers could achieve very high temperatures in their furnaces. He took strips of bubbled steel and placed them in a ceramic crucible, then placed the container in the glassblowing furnace. As a result, the steel melted, the iron silicate evaporated, but the carbon remained, resulting in very high quality steel. At that time, many people were watching the process, and he could not keep it a secret.
This method produced cast steel, from which a large number of old tools were made in the United States, labeled "cast steel."
Meteoric iron
The first iron that fell into the hands of man was not of terrestrial, but of cosmic origin: iron was part of meteorites falling to the Earth. Therefore, the Sumerians called it “heavenly copper”, and the ancient Copts called it “heavenly stone”. During the era of the first dynasties of Ur in Mesopotamia, iron was called an-bar (heavenly iron). The Egyptians always depicted iron objects as blue, the color of the sky. The Ebers Papyrus (previously 1500 BC) speaks of it as a metal of heavenly manufacture.
The largest iron meteorite was found in 1920 in southwestern Africa. This is the Goba meteorite, weighing 60 tons.
The fact that ancient people initially used iron of meteorite origin is evidenced by the widespread myths among some peoples about gods who dropped iron objects and tools from the sky - plows, axes. Meteoric iron is cold forged, so people began to make simple tools from it. Meteoric iron was processed in the same way as copper. During cold forging, it acquires the desired shape and at the same time becomes stronger and harder, and annealing in fire makes the forged metal soft again.
Iron production: features of smelting and extraction of raw materials
Many people mistakenly consider them cast, as the name suggests.
Steel production received a new impetus when the Bessemer steelmaking process was invented. This steel was used to build large projects, such as the Grand Coulee Dam, because it is not susceptible to corrosion. At the beginning of the 20th century, the production of various alloys began. Then, in gas open-hearth furnaces, manganese, chromium, nickel and other elements began to be added to iron.
During World War II, when the need for metal increased, the production of alloys received a new powerful impetus. Since then, huge strides have been made in the production and improvement of various steels.
Steel has higher physical and mechanical properties compared to cast iron: it can be forged, rolled, it has high strength and significant ductility, and is well processed by cutting.
In the molten state, steel has sufficient fluidity to produce castings.
Mild steel with a carbon content of less than 0.25% has high ductility, good weldability, and is easily forged and rolled in hot and cold states.
Therefore, such steel is the main material for modern mechanical engineering, transport and other sectors of the country’s national economy.
In ancient times, mild steel (technical iron) was obtained directly from ores in a doughy state. Later they learned to produce steel from cast iron in a brick forge, also in a dough-like state.
In 1740, a method for producing liquid steel in crucibles, known long before in the East, began to be used in England. Since 1784, they began to use puddling - producing steel in a dough-like state from cast iron by oxidizing its impurities on the hearth of a fiery furnace. All these methods were not very productive and required large amounts of fuel and labor.
The rapid growth of industry and railway transport in the second half of the 19th century. required a huge amount of steel, and the old methods of producing it could not satisfy this need. New, more productive methods for melting steel were created. In 1856, the Bessemer method appeared (named after its inventor G.
Bessemer), and in 1878 - the Thomas method (proposed by S. Thomas) of producing cast steel from liquid cast iron in converters. In 1857, the prominent Russian metallurgist P. M. Obukhov received the privilege of a method he invented for the production of gun steel by alloying cast iron and mild steel.
P. M. Obukhov's gun steel was superior in quality to the best foreign steels. Since 1864, the open-hearth method of producing steel in fiery furnaces (named after its inventor P. Martin) has been used, and since 1899, a method of producing steel in electric furnaces, based on the use of the phenomenon of an electric arc, discovered in 1802 by Acad. V.V. Petrov.
The task of converting cast iron into steel is to remove excess carbon, silicon, and manganese
and other impurities.
It is especially important to remove harmful impurities of sulfur and phosphorus
.
The carbon in cast iron combines with oxygen to form a gas (carbon monoxide CO), which evaporates. Other impurities are converted into oxides and other compounds that are insoluble or slightly soluble in the metal; These compounds, together with fluxes, form slag on the metal surface. When burned, manganese and silicon form metal-insoluble oxides MnO and SiO2. When phosphorus burns, its oxide P2O5 is formed, which is highly soluble in the metal.
To remove phosphorus from the metal, the slag is mixed with excess lime (consisting mainly of CaO), which binds P2O5 into a strong compound (CaO)4 • P2O5, insoluble in the metal.
Sulfur is dissolved in cast iron as part of the FeS compound; it is removed from the metal with the help of manganese or lime, which form with it either the MnS compound, which is poorly soluble in the metal, or the insoluble CaS compound.
Currently, the following methods of steel production are used in the country's metallurgy: converter, open-hearth and electric smelting.
Electric smelting is used mainly to produce high-quality steel and has been rapidly developing in recent years.
Technical progress in steelmaking is characterized by an intensive increase in the capacity of melting units, the widespread use of the oxygen-converter process and continuous casting of steel, and an increase in the quality of the metal.
History of iron
The Iron Age (1st millennium BC) is a period of early human history, which is determined by the development of metallurgy and the use of iron products (knives, axes, dishes, weapons, jewelry, etc.).
Iron Age in the system of three periods
The division of early human history into three periods of archaeological cultures: the Stone, Bronze and Iron Ages was proposed by the Danish archaeologist Christian Jurgensen Thomsen to facilitate the classification of archaeological finds.
Momsen's classification of artifacts works better for archaeological finds from the Mediterranean and the Middle East. In other ancient cultures, for example, the culture of Ancient China, it is more difficult to distinguish between the Bronze and Iron Ages. The term “Iron Age” appears much earlier, in the book “Works and Days” by Hesiod, where the history of mankind is divided into 5 eras: the Golden, Silver, Bronze, Age of Heroes and the Iron Age.
However, this ancient division is mythological, not archaeological. All peoples and civilizations experienced the period of spread of metallurgy and iron products. But Iron Age cultures include only civilizations of early history that subsequently went through a slave period.
Duration of the Iron Age
The period of the Iron Age was the shortest among other eras.
It began with the Dark Ages of Greece in the 12th century BC. in Europe and the Middle East, and in the 11th century in India and Asia. It is believed that the Iron Age ended with the emergence of written history around the 3rd century BC, which gives us an idea of the events from its direct participants (advanced Hellenism and the Roman state).
In America, Australia and Oceania, the Iron Age began only with the advent of Europeans.
So to speak, we continue to live during the developed Iron Age.
Iron and metallurgy have not lost their importance to this day. Until recently, the USSR was an incomparable leader in the production of iron and steel.
Discovery of iron
Early technology for obtaining and processing iron was primitive compared to modern metalworking. The oldest iron artifacts found by archaeologists were made from meteorite iron, or rather an alloy of iron and nickel.
Iron ore mining and iron smelting began at the end of the Bronze Age. The question of where this process began: whether there was first one center for iron smelting or whether this technology arose in different parts of the world independently is debated by archaeologists.
The most common theory states that iron smelting began in Eastern Anatolia around 1200 BC.
The first technology for melting iron was cheese blowing. A hole was dug in the ground, where ore and coal were piled in layers. A dome with a chimney was built over the pit. Air was supplied to the furnace using bellows. This design ensured the renewal of iron without melting - the temperature was too low.
The technology was ineffective. As a result, after destroying the furnace, a porous substance called steel was removed from it.
It consisted of iron and slag. It was then compacted using forge hammers. The raw iron was of low quality and brittle. It was inferior in hardness to bronze.
The advantage of iron over bronze was the availability of raw materials.
Ironmongery became better than bronze only with the beginning of the development of the steel-melting process, which occurred in the early Middle Ages. Since then, people began to widely use iron. Before that, hardware was inferior in quality to bronze, but iron ore was available and could be found almost everywhere, while the production of bronze requires copper and tin ores, the deposits of which were far away and required transportation and trade.
With the invention of iron smelting technology, significant changes occurred in human society - people received a sufficient number of tools.
Almost all household hardware, except scissors and screws, was first made in the Iron Age.
The mystery of the ancient column
In Delhi, there is the famous Qutub Column, weighing about 6.5 tons, its height is 7.5 m, its diameter is 42 cm at the base and up to 30 cm at the top. It is made of almost pure iron (99.72%), which explains its longevity. So far no rust has been found on it. The column was erected in 415 in honor of King Chandragupta II. According to popular belief, whoever leans his back against the column and clasps his hands behind it will have his cherished wish come true.
How were ancient metallurgists able to produce this wonderful column, before which time is powerless? Ancient India has long been famous for the skill of its metallurgists. Iron smelting in India is mentioned in the Rig Vedas - sacred books dating back to approximately the 13th-12th centuries. BC e. Thus, by the time the column was created, metallurgy in India had at least a thousand-and-a-half-year history, and iron had already begun to be used for the manufacture of plows.
There is still no consensus on the method of manufacturing this remarkable column. Some authors believe that the column was made by welding individual ends weighing 36 kg each and then forging them. According to other experts, ancient metallurgists, to obtain pure iron, ground a sponge of wrought iron into powder and sifted it. And then the resulting pure iron powder was heated to red heat and under the blows of a hammer its particles stuck together into one whole - now this is called the method of powder metallurgy.
How they searched for iron ore
In the middle of the century, metal products were highly valued, they were cherished and also passed on by inheritance.
The path to becoming a cauldron or an ax in those days was very long and long: iron had to be found and then processed.
The business began with a search for places where metal ores lay. The search was helped by the experience that people have accumulated over many centuries. First of all, these are deposits that reach the surface of the earth.
Throughout Europe, iron was found in the form of lumps of ore:
1. greenish - at the bottom of lakes;
2. reddish - under the turf;
3. reddish - in forest swamps.
The bottom of the clear lakes was scanned from boats, or they dived into the muddy water in search of pieces of ore, which they scooped out with scoops.
Iron ore was also found in brown vegetation. The meadow turf was cut, the swamp layers were torn off, and the ore nest was taken out with shovels. Sometimes such a meadow was covered with thousands of holes.
A little later, ore began to be mined in mines that reached depths of up to 500 meters.
Iron ore was lifted from the mines using lifting mechanisms, and groundwater was pumped out with hand pumps.