Characteristics of types of carbon metal
The iron-carbon diagram shows what cast iron is made of. In addition to iron, carbon is present in the form of graphite and cementite.
The composition of the cast iron alloy has varieties:
- White. The carbon present here is in a chemically bound state. The metal is strong, but brittle and therefore difficult to machine. In industry it is used in the form of castings. The properties of the material allow it to be processed with an abrasive wheel. The welding process causes difficulty, since there is a possibility of cracks due to the heterogeneity of the structure. Found application in areas related to dry friction. Has increased heat resistance and wear resistance.
- Half-hearted. It has increased fragility, so it is not widely used.
- Grey. GOST 1412–85 indicates what percentage of impurities this metal contains: 3.5% carbon, 0.8% manganese, 0.3% phosphorus, 0.12% sulfur and up to 2.5% silicon. The carbon present in the platelet form creates low impact strength. The characteristics of the type indicate that the material works better in compression than in tension. When heated sufficiently, it has good weldability.
- Malleable. A ferrite base of this type provides it with high ductility. When broken, it has a black, velvety color. It is obtained from white, which languishes for a long time at a temperature of 800-950 degrees.
- Highly durable. The difference from other types is the presence of spherical graphite. It is obtained from gray after adding magnesium to it.
Open hearth method
This method is used to produce high-quality steels used in critical machine parts and precision mechanisms.
At one time, it replaced the labor-intensive and low-productivity crucible and pulding melts that had been used previously.
The loading capacity of one reverberatory furnace used in this method reaches 500 tons. A special feature of the open-hearth method is the ability to remelt not only pig iron, but also metallurgical waste and scrap metal.
The heating temperature of liquid steel reaches 2 thousand degrees. This result is achieved by the special design of the open hearth furnace:
- the use of additional heat from regenerators obtained by burning coke oven or blast furnace gas in a stream of hot air;
- reflections from the roof of the injected gas as a result of fuel combustion in it occurring above a bath of metal, which contributes to the rapid heating of the contents;
- using reversal of the heating flow.
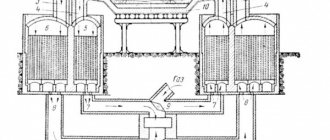
An open hearth furnace consists of the following elements:
- working space with fire-resistant lining of walls and filling windows;
- hearths (bases) made of magnesite brick;
- furnace roof;
- furnace heads;
- slag for removing dust;
- regenerator with changeover valves.
The smelting process takes from 4 to 12 hours. In order to speed up the smelting process, the volume of pumped oxygen exceeds the requirements, which increases smelting productivity by 20–30%.
Individual properties of metal
The material is characterized by certain characteristics. These include:
- Physical. Values such as specific gravity or expansion coefficient depend on the carbon content of the metal. The material is heavy, so cast iron bathtubs can be made from it.
- Thermal. Thermal conductivity allows you to accumulate heat and retain it, distributing it evenly in all directions. This is used in the manufacture of frying pans or radiators for heating.
- Mechanical. These characteristics vary depending on the graphite base. The most durable is gray cast iron with a perlite base. A material with a ferrite component is more malleable.
Depending on the presence of impurities, a difference in the properties of the material appears.
These elements include sulfur, phosphorus, silicon, manganese:
- Sulfur reduces the fluidity of the metal.
- Phosphorus reduces strength, but makes it possible to manufacture products of complex shapes.
- Silicon increases the fluidity of the material, lowering its melting point.
- Manganese gives strength, but reduces fluidity.
Differences between cast iron and steel
To understand the difference between steel and cast iron, you need to consider their characteristics. A distinctive feature of cast iron is the amount of carbon. Its minimum content is 2.14%. This is the main indicator by which this material can be distinguished from steel.
The iron content in steel is 45%, and the percentage of carbon is up to 2. To determine the differences by eye, you need to pay attention to the color. Steel is light in color and cast iron is dark.
Only chemical analysis can determine the percentage of impurities. If we compare the melting point of cast iron and steel, then for cast iron it is lower and amounts to 1150-1250 degrees. For steel - around 1500.
To distinguish the material, you need to do the following:
- The product is lowered into water and the volume of displaced water is determined. Cast iron has a lower density. It is 7.2 g/cm3. For steel - 7.7−7.9 g/cm3.
- A magnet is applied to the surface, which attracts the steel better.
- The chips are rubbed using a grinder or file. Then it is collected in paper and wiped on it. Steel will not leave marks.
Pros and cons of the material
Like any material, cast iron has positive and negative sides. Positive qualities include:
- a wide variety of conditions.
- some types are highly durable;
- the ability to maintain temperature for a long time;
- environmentally friendly, which allows you to make dishes from it;
- resistance to acid-base environment;
- high hygiene;
- long service life and durability;
- harmlessness of the material.
Read also: Pke 722 2у2 connection diagram
However, there are also disadvantages. These include:
- when kept in water for a long time, the surface becomes covered with rust;
- high cost of material;
- low ductility of gray cast iron;
- fragility.
Cast iron is a metal characterized by a high carbon content. Thanks to this, it has qualities that are necessary for industrial and domestic purposes.
Installations for automatic welding of longitudinal seams of shells - in stock!
High performance, convenience, ease of operation and reliability in operation.
Welding screens and protective curtains are in stock!
Radiation protection when welding and cutting. Big choice. Delivery throughout Russia!
Metals and alloys according to their chemical composition are divided into non-ferrous (copper, aluminum, lead, bronze, brass, etc.) and ferrous (iron, steel, cast iron). Metals are rarely used in their pure form, but mostly in the form of alloys.
Cast iron and steel are alloys of iron and carbon, in which the presence of impurities of other chemical elements is inevitable:
Steel: Fe + C (2%) + impurities (more than steel).
What are the similarities and differences between these alloys?
The basis is one - iron. The main difference is that cast iron has a high carbon content (over 2% in cast iron and up to 2% in steel). The boundary between these alloys is based on the carbon content in the alloy. Many cast irons also contain more manganese, sulfur, phosphorus and silicon.
Steel is often harder, stronger and more wear-resistant. Cast iron is more brittle, but has good casting properties. Steel is a derivative of cast iron because... Its production is mainly two-stage: first, cast iron is obtained from iron ores, then steel is obtained from cast iron and scrap steel.
Almost half of the world's proven iron reserves are located in the CIS countries. The former USSR produced and produced more iron and steel than anyone else in the world. The reasons for this “achievement” were: imperfect designs and low reliability of machinery and equipment; low quality of smelted cast iron and steel; huge territories; large length of roads and communications; low efficiency of agricultural production, construction and road work. All this required much more metal than in other countries. And, besides, there was more metal buried in the ground at construction sites, abandoned in landfills, in forests, swamps and fields than anyone else in the world.
Historically, the production of ferrous metals developed in the following stages:
- Cheese-making process (1500 BC). The productivity of the process is very low; in 1 hour only up to 0.5...0.6 kg of iron was obtained. In forges, iron was reduced from ore with coal when blown with air using forge bellows. First, when burning charcoal, carbon monoxide was formed, which reduced pure iron from the ore.
As a result of long-term air blowing from pieces of ore, pieces of pure iron were obtained, practically free of impurities, which were welded together using a forge into strips, which were then used to produce products necessary for humans. This technically pure iron contained very little carbon and few impurities (pure charcoal and good ore), so it forged and welded well and practically did not corrode. The process took place at a relatively low temperature (up to 1100...1350 o C), the metal did not melt, i.e., the reduction of the metal took place in the solid phase. The result was malleable iron. This method existed until the 14th century, and in a slightly improved form until the beginning of the 20th century, but was gradually replaced by critical redistribution.
It follows that historically the very first metal welder was a blacksmith, and the very first welding method was forge welding.
- With the increase in the size of the cheese furnaces and the intensification of the process, the carbon content in iron increased, the melting point of this alloy (cast iron) turned out to be lower than that of purer iron, and part of the metal was obtained in the form of molten cast iron, which, as a production waste, flowed out of the furnace along with the slag. In the 14th century, a two-stage method for producing iron was developed in Europe (a small blast furnace, then a furnace process). Productivity increased to 40...50 kg/hour of iron. A water wheel was used to supply air. Critical processing
is the process of refining cast iron (reducing the amount of C, Si, Mn) in order to obtain cast iron (welding) iron. - At the end of the 18th century, mineral fuels began to be used in Europe in the blast furnace process and in the puddling process
. In the puddling process, coal is burned in a furnace, gas passes through the bath, melts and purifies the metal. In China, even earlier, in the 10th century, cast iron was smelted, and then steel was obtained by the process of puddling. Pudding is the cleaning of cast iron in a fiery furnace. During cleaning, iron grains collect into clumps. The pudliner turns the mass over and over with a crowbar and divides it into 3...5 parts - krits. In a forge or rolling machine, grains are welded to produce strips and other blanks. Steam engines are already used instead of a water wheel. Productivity increases to 140 kg of wrought iron per hour. - At the end of the 19th century, three new processes for producing steel were introduced almost simultaneously: Bessemer, open-hearth and Thomas. Steel melting productivity increases sharply (up to 6 tons/hour).
- In the middle of the 20th century: oxygen blasting, process automation and continuous casting of steel were introduced.
Read also: Sectional design of a propane gas cylinder
During the cheese-blowing, krichny and puddling processes, iron did not melt (the technical level of that time did not make it possible to ensure its melting temperature). Blowing oxygen through the molten metal in a Bessemer converter, due to the sharp increase in the contact surface of the metal with the oxidizing agent (oxygen), accelerates chemical reactions a thousand times compared to a puddling furnace.
In the cheese-blowing and casting processes, malleable, wrought iron (low-carbon steel) was obtained in a single-stage method, which had a small amount of impurities and was therefore very resistant to corrosion. Currently, a one-stage steel production process is under development: ore beneficiation (production of pellets containing 90...95% iron) and steel smelting in an electric furnace.
Iron Ore for Steel
The process of steel smelting begins with the processing of iron ore. The rock containing the iron ore is crushed. The ore is mined using magnetic rollers. Fine-grained iron ore is processed into coarse-grained lumps for use in the blast furnace. Coal is purified of impurities in a coke oven, resulting in a nearly pure form of carbon. The mixture of iron ore and coal is then heated to produce molten iron or pig iron, which is used to make steel.
In the main oxygen furnace, molten iron ore is the main raw material and is mixed with varying amounts of scrap steel and alloys to produce different grades of steel. An electric arc furnace melts recycled steel scrap directly into new steel. About 12% of steel is made from recycled material.
Iron production
Cast iron is smelted in blast furnaces. This is a complex engineering structure that operates continuously for 5..10 years.
The oven operates on the counterflow principle. Ore, fluxes and coke are loaded from above, and air is supplied from below. Coke serves to heat and melt ore, and also participates in the reduction of iron from ore oxides. Coke should contain a minimum of sulfur and phosphorus. Fluxes (limestones, silicas) are necessary for the production of slags. When fuel is burned, carbon monoxide is formed, which is the main reducing agent for iron.
Steel production
To obtain steel from cast iron, it is necessary to reduce the amount of carbon, manganese, sulfur and phosphorus in it. Steel is produced in oxygen converters, open-hearth furnaces and electric furnaces.
Open hearth production is less productive than converter production, but the process is better regulated; pig iron and scrap metal are used. Martin is a regenerative combustion furnace. The gas burns above the melting space, where a temperature of 1750...1800 o C is created. Gas and air are preheated (up to 1200...1250 o C) in regenerators. Due to the heat of burnt gases escaping into the pipe. Two regenerators: one works, and the other accumulates thermal energy. To intensify the process, the bath is purged with oxygen. The deoxidation of the bath is carried out with ferrosilicon and ferromanganese in the bath, and the final deoxidation is carried out with aluminum and ferrosilicon in a steel-pouring ladle.
High quality steel is smelted in arc and induction electric furnaces. The process is approximately the same as in an open-hearth furnace, but the temperature is higher, so it is possible to produce refractory steel containing chromium, tungsten, etc. in electric furnaces. Two periods during the smelting of electric steel: oxidative (Si, Mn, C, Fe burn out) due to oxygen, air and charge oxides; reducing - deoxidation of steel, removal of sulfur. To do this, a flux consisting of lime and fluorspar is introduced.
Induction melting is usually used for remelting steels and producing high-alloy and special steels under vacuum or a special controlled atmosphere.
Source: N.V. Khramtsov. Metals and welding (lecture course)
Read also: How many buckets in a cube of concrete
Steel remains the main structural material for construction, engineering and many other industries. The separation of iron-carbon alloys depends on the carbon content. Conventionally, we can assume that with a carbon content of up to 2% it is steel, and more than 2% is cast iron. In the literature, in the works of the great materials scientist Gulyaev, it is clarified that the interface of 2.14% is valid only with a negligible content of any other elements except iron and carbon. It is not surprising that it is used in production at large plants and small foundries.
The use of high-carbon alloys in steelmaking can be done cold. It has the shape of a pyramid, previously smelted at metallurgical plants from ore and fluxing materials and poured on special casting machines. The advantage of using this type of raw material is unlimited logistics and guaranteed chemical analysis of such material. This allows steelmaking shops to carry out preliminary preparatory work to combat sulfur, phosphorus and other elements that negatively affect the physical properties of the finished product.
Large metallurgical plants with a full cycle use cast iron in liquid form for steel smelting. Before steel is made from cast iron, it is produced in a blast furnace shop. During release from the blast furnace, the pig iron is poured into ladles, protected by a liner from high temperatures. In these ladles it is brought to the steelmaking shop, where it is poured into a large container in which up to 1800 tons of pig iron from different blast furnaces are mixed. This container is called a mixer. It is used for averaging of chemical analysis and temperature indicator. Many factories use mixer buckets. At the command of the furnace steelmaker, converter or shift supervisor, a weighed portion in buckets is dispensed from the mixer for the next stage.
By this time, the steel-smelting unit already contains scrap steel heated to a certain temperature. Cast iron is poured onto this scrap. Further adding and removing slag, changing its basicity and temperature, the steelmaker brings the melt to the required temperature and chemical composition. This production process saves a large amount of energy and reduces production costs on a production scale.
The main units for using cast iron in steel production are converters, open-hearth furnaces, electric arc furnaces, and induction furnaces.
In open hearth furnaces, a heat recovery process is implemented. A stream of hot air passing over the molten bath of the furnace heats up and heats the regenerators. After a certain period of time, the direction of the air flow changes and, in contact with the regenerators, it heats up. Higher air temperatures increase the calorific value of calorific gas combustion. Invented in 1864 by Pierre Martin, this unit is today considered a turning page in the history of metallurgy. Operating open-hearth furnaces are not able to cope with competition and the requirements for the steel produced.
A converter is a unit in which scrap and pig iron in liquid form are blown with oxygen or air. Carbon oxidation occurs with additional heat release. Thus, two tasks are solved simultaneously - reducing the carbon equivalent and achieving temperature indicators sufficient to combat sulfur and a number of other elements. More than half of the world's steel is produced in converters.
An electric arc furnace is an integral element in the production of stainless, alloy, and specialized steels. The heat in this furnace is induced by an electric arc that occurs between the electrodes and the metal bath. Cast iron is placed in the fill or poured after preheating the metal. Next, fluxing materials and slag blowing agents are added. This allows you to remove sulfur and phosphorus from the metal.
In an induction furnace, cast iron is primarily used as a cold charging material in the smelting of steel. The inability to influence the content of sulfur and phosphorus when melting in such a furnace places particularly high demands on the content of these elements on cast iron.
The production of steel from cast iron is justified in large-scale production and small foundries and sites.
In oxygen converters
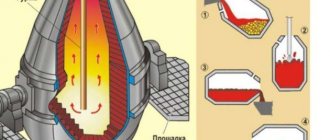
Today, various steels are produced in oxygen converters. This technology involves purging liquid cast iron in a converter. To do this, pure oxygen is supplied. The features of this technology include the following points:
- A converter is a special equipment, which is represented by a pear-shaped steel vessel. The capacity of such a device is 100-350 tons. The inside of the structure is lined with refractory bricks.
- The design of the upper part includes a neck, which is necessary for loading the charge and liquid cast iron. In addition, gases formed during the melting of raw materials are removed through the neck.
- Pouring cast iron and adding other charge is carried out at a temperature of about 1400 degrees Celsius. In order to ensure active oxidation of iron, pure oxygen is supplied under a pressure of about 1.4 MPa.
- When a large amount of oxygen is supplied, cast iron and other mixtures oxidize, which causes the release of a large amount of heat. Due to strong heating, the entire charge material melts.
- At the moment when excess carbon is removed from the composition, the blowing stops and the lance is removed from the converter. Typically, purging continues for 20 minutes.
- At this stage, the resulting composition contains a large amount of oxygen. That is why, to improve performance, various deoxidizing agents and alloying elements are added to the composition. The resulting slag is removed into a special slag ladle.
- Converter melting time may vary, as a rule, it is 35-60 minutes. The holding time depends on the type of charge used and the volume of steel produced.
Oxygen converter method
It is worth considering that the productivity of such equipment is about 1.5 million tons with a capacity of 250 tons. This technology is used to produce carbon, low-carbon, and alloy steels. The oxygen-converter method of steel production was developed quite a long time ago, but today it is still very popular. This is due to the fact that when using this technology, high-quality metals can be obtained, and the productivity of the technology is very high.
In conclusion, we note that it is almost impossible to produce steel at home. This is due to the need to heat the charge to a sufficiently high temperature. At the same time, the process of iron oxidation is very complex, as is the removal of harmful impurities