Before talking about the melting point of stainless steel, it is worth noting that this physical characteristic is important for foundries, welders, and manufacturers of branded stainless steel.
When metalworking, other concepts are used, for example, the point of eutectic (equilibrium of the liquid and solid phases), the point of plasticity (t, at which the alloy becomes soft and pliable).
Metal melting
Solder for stainless steel
The melting point of tin largely depends on whether there are impurities. The temperature at which the metal becomes plastic or liquid can vary from 145 to 250 degrees Celsius, depending on the composition. If necessary, you can melt a large amount of metal to fill it into a mold.
When choosing a material to create a mold, the following points are taken into account:
- The structure should not be wetted by liquid tin. Otherwise, the form may change its size.
- The material used must withstand temperatures of at least 250 degrees Celsius. Otherwise, after filling, the mold will lose its basic performance qualities.
It is worth considering that in liquid form the metal in question can oxidize upon contact with air. The solid substance, on the contrary, has increased resistance to oxygen corrosion.
A three-component alloy based on lead has become quite widespread in electrical engineering. Tin and silver can be used as additional components
When producing such an alloy, attention is paid to the fact that the metal concentration should not be less than 95%. With this option, the combination of substances has a melting point of about 220 degrees Celsius
Metal classification
Man has long known the melting temperatures of metals and alloys. Thanks to this data, they can be divided into three large groups:
- Low-melting metals - melt up to 600 degrees Celsius. These include tin, zinc, and lead.
- Medium melting - melts in the range of 600-1600 degrees Celsius. The most extensive group, which includes all possible alloys and homogeneous materials.
- Refractory - melts at 1600 degrees Celsius. These include titanium, chromium, molybdenum, tungsten.
To find out more accurate information, you can study the table of melting temperatures of metals. You can find it on the Internet or special reference books for foundry workers. The heat of fusion of alloys depends on the amount of impurities contained in the composition.
Features of material classification
Melting temperature of solder. properties of solders and bearing materials
The material is more brittle compared to steel and can break even in cases where there is no significant deformation. Carbon in the composition is presented in the form of graphite or cementite; each substance can be presented separately. Cast iron is divided into types, focusing on the shape and quantity of these substances:
- White. Carbon in its entirety is in the form of cementite. The tint can be seen precisely on the fracture of the materials. It is distinguished by fragility and simultaneous hardness. It can be processed mainly in order to ensure normal forging.
- Grey. Carbon has a plastic form in the form of graphite. It is characterized by softness and is easy to process at low temperatures.
- Malleable. This designation is conditional, because the material cannot be forged. The variety is obtained by prolonged firing of white, resulting in the formation of graphite. The beneficial properties are negatively affected by heating exceeding 900 degrees Celsius, as well as a significant cooling rate of the graphite itself, which leads to difficulties in the processing and welding process.
- Highly durable. It is characterized by the content of spherical graphite, which is obtained by crystallization.
Differences from steel
The difference between the two materials is as follows:
- Cast iron has less hardness and strength compared to steel.
- Steel weighs more and has a higher melting point.
- A small percentage of carbon in steel makes it malleable to various types of processing (forging, cutting, welding, rolling). For this reason, cast iron products are made by casting.
- Decorative steel products have a beautiful shine, while those made of cast iron are matte with a black tint.
- Cast iron is the primary product of ferrous metallurgy, steel is the final product.
- The steel is hardened.
- Cast iron products are produced by casting, steel products by forging and welding.
Features of the steel production process
In the production of cast iron and steel, different technologies are used, despite the fairly similar chemical composition and some physical and mechanical properties. The differences are that steel contains less harmful impurities and carbon, due to which high performance is achieved. During the smelting process, all impurities and excess carbon, which causes an increase in the fragility of the material, go into slag. Steel production technology involves forced oxidation of basic elements due to the interaction of iron with oxygen.
When considering the production process of carbon and other types of steel, several main stages of the process should be highlighted:
- Rock melting. The raw materials that are used to produce metal are called charge. At this stage, during the oxidation of iron, impurities are also deoxidized. Much attention is paid to reducing the concentration of harmful impurities, which include phosphorus. To ensure the most suitable conditions for the oxidation of harmful impurities, a relatively low temperature is initially maintained. The formation of iron slag occurs by adding iron ore. After the release of harmful impurities on the surface of the alloy, they are removed, and a new portion of calcium oxide is added.
- Boiling of the resulting mass. After the preliminary stage of cleaning the composition, baths of molten metal are heated to a high temperature, and the alloy begins to boil. Due to boiling, the carbon contained in the composition begins to actively oxidize. As previously noted, cast iron differs from steel in having too high a carbon concentration, due to which the material becomes brittle and acquires other properties. This problem can be solved by injecting pure oxygen, due to which the oxidation process will occur at high speed. When boiling, bubbles of carbon monoxide are formed, to which other impurities also adhere, due to which the composition is purified. At this stage of production, sulfur, which is a harmful impurity, is removed from the composition.
- Deoxidation of the composition. On the one hand, adding oxygen to the composition ensures the removal of harmful impurities, on the other hand, it leads to a deterioration in basic performance qualities. That is why, to clean the composition from harmful impurities, diffusion deoxidation is often carried out, which is based on the introduction of a special molten metal. This material contains substances that have approximately the same effect on the molten alloy as oxygen.
In addition, depending on the characteristics of the technology used, two types of materials can be obtained:
- Calm ones who have gone through the deoxidation process to the end.
- Semi-quiet, which have a state between calm and boiling steels.
Melting iron
Stainless steel grades and their characteristics
What determines the melting point of iron?
Metal production involves various technologies for its extraction from ore raw materials. The most common method for smelting iron is the blast furnace method.
In addition to the blast furnace method, iron smelting is carried out by roasting crushed ore with clay. The mixture is formed into pellets and processed in a hydrogen reduction furnace. Further smelting of iron is carried out in electric furnaces.
Production of alloys in furnaces.
The properties of a metal depend on the purity of the material. For technically pure iron, the melting point is +1539 °C. Sulfur is a harmful impurity. It can only be extracted from a liquid solution. Chemically pure material is obtained by electrolysis of metal salts.
Metal alloys
In its pure form, this material is soft, so carbon is added to the composition to increase strength.
Depending on the components of the alloy, the properties of the materials change. The melting point of iron also changes in the presence of alloy components.
The specific heat of fusion of steel is 84 kJ. This indicator means that at the melting temperature of steel, 84 kJ of energy is required to transfer 1 kg of alloy from a crystalline to a liquid state.
Compounds of different metals form alloys. The specific heat of fusion of cast iron is 96–140 kJ. Cast iron contains up to 4% carbon, 1.5% manganese, up to 4.5% silicon and impurities in the form of sulfur and phosphorus. There are white and gray alloys.
In white, part of the carbon is in the iron carbide compound. This alloy is brittle and hard. It is intended for the manufacture of structures and parts.
A gray alloy containing carbon in the form of graphite, it is easy to process. Pig iron is smelted from iron ore in blast furnaces. Melting of the ore is accompanied by the reduction reaction of iron from oxides with carbon.
Most substances can melt with increasing volume when heated. For cast iron with a volume of 1000 cm³, this figure is 988–994 cm³.
Cast iron is a raw material for the production of steel, characterized by carbon content (not higher than 2.14%).
Steel is classified according to its chemical composition:
- alloyed;
- carbon
Carbon steel contains impurities of sulfur, phosphorus and silicon. It is characterized by low electrical properties, low strength, and is easily susceptible to corrosion.
The presence of alloy additives gives steel new technical properties. The following are used as additional components:
- molybdenum;
- nickel;
- tungsten;
- chromium;
- vanadium.
High-alloy steel contains no more than 10% additives. The alloy is durable. The technology for producing steel from cast iron allows us to obtain high-quality material for the production of:
- metal structures;
- tanks;
- dishes;
- reinforcing parts;
- electrical equipment.
Iron is widely used in all areas of human life.
Cast iron and steel
Cast iron is an alloy of carbon and iron, it contains impurities of manganese, silicon, sulfur and phosphorus. Withstands low voltages and loads. One of its many advantages is its low cost for consumers. There are four types of cast iron:
- White - has high strength and poor ability to be processed with a knife. Types of alloy according to the increase in the amount of carbon in the composition: hypoeutectic, eutectic, hypereutectic. It was called white due to the fact that it has a white color in the fault. White cast iron also has a special structure of the metal mass and great wear resistance. Useful in making mechanical parts that will operate in a non-lubricated environment. It is used to make the following types of cast iron.
- Gray cast iron - contains carbon, silicon, manganese, phosphorus and some sulfur. It can be easily obtained and has poor mechanical properties. Used for the manufacture of parts that are not exposed to shock loads. There is a gray color in the fracture; the darker it is, the softer the material. The properties of gray cast iron depend on the temperature of the environment in which it is located and the amount of various impurities.
- Malleable cast iron is obtained from white cast iron as a result of simmering (prolonged heating and holding). The substance contains: carbon, silicon, manganese, phosphorus, and a small amount of sulfur. It is more durable and ductile, easier to process.
- Ductile iron is the strongest of all types of cast iron. Contains carbon, manganese, sulfur, phosphorus, silicon. Has high impact strength. This important metal is used to make pistons, crankshafts and pipes.
The melting points of steel and cast iron are different, as stated in the table above. Steel has higher strength and resistance to high temperatures than cast iron, temperatures differ by as much as 200 degrees. For cast iron, this number ranges from approximately 1100 to 1200 degrees, depending on the impurities it contains.
How the process works
Elements, whatever they are: gold, iron, cast iron, steel or any other, melt approximately the same. This occurs due to external or internal heating. External heating is carried out in a thermal furnace. For internal heating, resistive heating is used, passing an electric current or induction heating in a high-frequency electromagnetic field. The impact is approximately the same.
When heating occurs, the amplitude of thermal vibrations of molecules increases. Structural lattice defects appear, accompanied by the rupture of interatomic bonds. The period of lattice destruction and accumulation of defects is called melting.
Depending on the degree at which metals melt, they are divided into:
- low-melting - up to 600 °C: lead, zinc, tin;
- medium-melting - from 600 °C to 1600 °C: gold, copper, aluminum, cast iron, iron and most of all elements and compounds;
- refractory - from 1600 °C: chromium, tungsten, molybdenum, titanium.
Depending on what the maximum degree is, the melting apparatus is selected. It should be stronger the stronger the heating.
The second important value is the boiling degree. This is the parameter at which liquids begin to boil. As a rule, it is twice the melting point. These values are directly proportional to each other and are usually given at normal pressure.
If the pressure increases, the amount of melting also increases. If the pressure decreases, then it decreases.
Metal separation
Depending on their melting point, metals are divided into:
. This is zinc, lead, hang, tin.
Medium melting: melting point ranges from 600C o
. These are gold, copper, aluminum, magnesium, iron, nickel and more than half of all elements.
Refractory: requires temperatures above 1600C o
to make the metal liquid. These include chromium, tungsten, molybdenum, titanium.
Depending on the melting temperature, the melting apparatus is also selected . The higher the indicator, the stronger it should be. You can find out the temperature of the element you need from the table.
Another important quantity is the boiling point. This is the value at which the process of boiling liquids begins; it corresponds to the temperature of saturated steam that forms above the flat surface of the boiling liquid. It is usually almost twice the melting point.
Both values are usually given at normal pressure. directly proportional to each other .
- As the pressure increases, the amount of melting increases.
- As the pressure decreases, the amount of melting decreases.
Table of low-melting metals and alloys (up to 600C
| Item name | Latin designation | Temperatures | |
| Melting | Boiling | ||
| Tin | Sn | 232 C about | 2600 C about |
| Lead | Pb | 327 C about | 1750 C about |
| Zinc | Zn | 420 C o | 907 C o |
| Potassium | K | 63.6 C o | 759 C o |
| Sodium | Na | 97.8 C o | 883 C about |
| Mercury | Hg | — 38.9 C o | 356.73 C o |
| Cesium | Cs | 28.4 C o | 667.5 C o |
| Bismuth | Bi | 271.4 C o | 1564 C about |
| Palladium | Pd | 327.5 C o | 1749 C about |
| Polonium | Po | 254 C about | 962 C about |
| Cadmium | Cd | 321.07 C o | 767 C o |
| Rubidium | Rb | 39.3 C o | 688 C about |
| Gallium | Ga | 29.76 C o | 2204 C about |
| Indium | In | 156.6 C o | 2072 C about |
| Thallium | Tl | 304 C about | 1473 C about |
| Lithium | Li | 18.05 C o | 1342 C about |
Table of medium-melting metals and alloys (from 600C
| Item name | Latin designation | Temperatures | |
| Melting | Boiling | ||
| Aluminum | Al | 660 C o | 2519 C about |
| Germanium | Ge | 937 C about | 2830 C about |
| Magnesium | Mg | 650 C o | 1100 C about |
| Silver | Ag | 960 C o | 2180 C about |
| Gold | Au | 1063 C o | 2660 C about |
| Copper | Cu | 1083 C about | 2580 C about |
| Iron | Fe | 1539 C about | 2900 C about |
| Silicon | Si | 1415 C about | 2350 C about |
| Nickel | Ni | 1455 C about | 2913 C about |
| Barium | Ba | 727 C o | 1897 C about |
| Beryllium | Be | 1287 C about | 2471 C about |
| Neptunium | Np | 644 C o | 3901.85 C o |
| Protactinium | Pa | 1572 C about | 4027 C about |
| Plutonium | Pu | 640 C o | 3228 C about |
| Actinium | Ac | 1051 C o | 3198 C about |
| Calcium | Ca | 842 C about | 1484 C about |
| Radium | Ra | 700 C o | 1736.85 C o |
| Cobalt | Co | 1495 C about | 2927 C about |
| Antimony | Sb | 630.63 C o | 1587 C about |
| Strontium | Sr | 777 C about | 1382 C about |
| Uranus | U | 1135 C about | 4131 C about |
| Manganese | Mn | 1246 C about | 2061 C about |
| Konstantin | 1260 C about | ||
| Duralumin | Alloy of aluminum, magnesium, copper and manganese | 650 C o | |
| Invar | Nickel iron alloy | 1425 C about | |
| Brass | Copper and zinc alloy | 1000 C o | |
| Nickel silver | Alloy of copper, zinc and nickel | 1100 C about | |
| Nichrome | Alloy of nickel, chromium, silicon, iron, manganese and aluminum | 1400 C about | |
| Steel | Iron-carbon alloy | 1300 C about | |
| Fechral | Alloy of chromium, iron, aluminum, manganese and silicon | 1460 C about | |
| Cast iron | Iron-carbon alloy | 1100 C about | |
Table of refractory metals and alloys (over 1600C
| Item name | Latin designation | Temperatures | |
| Melting | Boiling | ||
| Tungsten | W | 3420 C about | 5555 C about |
| Titanium | Ti | 1680 C about | 3300 C about |
| Iridium | Ir | 2447 C about | 4428 C about |
| Osmium | Os | 3054 C about | 5012 C about |
| Platinum | Pt | 1769.3 C o | 3825 C about |
| Rhenium | Re | 3186 C about | 5596 C about |
| Chromium | Cr | 1907 C o | 2671 C about |
| Rhodium | Rh | 1964 C about | 3695 C about |
| Ruthenium | Ru | 2334 C about | 4150 C about |
| Hafnium | Hf | 2233 C about | 4603 C about |
| Tantalum | Ta | 3017 C about | 5458 C about |
| Technetium | Tc | 2157 C about | 4265 C about |
| Thorium | Th | 1750 C about | 4788 C about |
| Vanadium | V | 1910 C about | 3407 C about |
| Zirconium | Zr | 1855 C about | 4409 C about |
| Niobium | Nb | 2477 C about | 4744 C about |
| Molybdenum | Mo | 2623 C about | 4639 C about |
| Hafnium carbides | 3890 C about | ||
| Niobium carbides | 3760 C about | ||
| Titanium carbides | 3150 C about | ||
| Zirconium carbides | 3530 C about | ||
Melting iron
The melting point of iron is quite high. A technically pure element requires a temperature of +1539 °C. This substance contains an impurity - sulfur, and it can only be extracted in liquid form.
Without impurities, pure material can be obtained by electrolysis of metal salts.
Melting cast iron
Cast iron is the best metal for smelting. The high fluidity rate and low shrinkage rate make it possible to use it more effectively during casting. Next, consider the boiling point of cast iron in degrees Celsius:
- Gray - the temperature can reach 1260 degrees. When pouring into molds, the temperature can rise to 1400.
- White - the temperature reaches 1350 degrees. It is poured into molds at 1450.
Important! The melting rates of a metal such as cast iron are 400 degrees lower compared to steel. This significantly reduces energy costs during processing.
Melting steel
Melting steel at a temperature of 1400 °C
Steel is an alloy of iron with an admixture of carbon. Its main benefit is strength, since this substance is capable of maintaining its volume and shape for a long time. This is due to the fact that the particles are in an equilibrium position. Thus, the forces of attraction and repulsion between particles are equal.
Reference! Steel melts at 1400 °C.
Melting aluminum and copper
The melting point of aluminum is 660 degrees, which means that it can be melted at home.
Pure copper is 1083 degrees, and for copper alloys it ranges from 930 to 1140 degrees.
Metal alloys
To design products from alloys, their properties are first studied. To study, the metals being studied are melted in small containers in different ratios to each other. Based on the results, graphs are built.
The lower axis represents the concentration of component A with component B. The vertical axis is temperature. Here the values of the maximum temperature are noted when all the metal is in a molten state.
When cooled, one of the components begins to form crystals. In a liquid state, eutectic is an ideal compound of metals in an alloy.
Metallurgists identify a special ratio of components at which the melting point is minimal. When making alloys, they try to select the amount of substances used in order to obtain a eutectoid alloy. Its mechanical properties are the best possible. Crystal lattices form ideal face-centered positions of atoms.
The crystallization process is studied by studying the hardening of samples upon cooling. They build special graphs where they observe how the cooling rate changes. Ready-made diagrams are available for different alloys. By marking the start and end points of crystallization, the composition of the alloy is determined.
Alloy types
Depending on the intensity of heating required for the transition of a metal from one state to another, alloys are divided into several types.
Low fusible. They can be processed even without special equipment. The melting point of steel in degrees Celsius is 600. Low-melting metals include lead, tin and zinc.
Mercury deserves special attention because it can turn into a liquid state at -39°C.
Medium melting. The melting point of steel is in the range of 600°C-1600°C. This category includes aluminum, copper, tin, some types of stainless steel and various alloys with a small chromium content. Medium-melting compounds are most widespread in industry and in everyday life.
Refractory. Compounds included in this category are capable of transitioning from solid to liquid when heated above 1600°C. These are highly alloyed metals, which include tungsten, titanium and chromium. Thanks to these additives, the metal acquires increased strength, resistance to corrosion and chemical influences. In particular, stainless steel is a refractory alloy.
Alkali metals melt at the lowest temperatures. Accordingly, for non-alkali metals to transition into the liquid state, the temperature range increases significantly.
Boiling degree
When heating a material, it is important not to reach its boiling point, at which point it goes from a liquid state to a gaseous state. Therefore, the boiling point is an equally important technological indicator.
The boiling point is typically twice the degree at which materials melt and is determined at normal atmospheric pressure. As the pressure increases, the heating intensity also increases. As the pressure decreases, the readings decrease.
Features of Carbon Steel
Carbon compounds are the main type of products produced at metallurgical plants. In addition to iron, they also contain carbon. Its concentration should not exceed 2.14%. They contain a small amount of impurities and alloying components in the form of manganese, silicon and magnesium. Such additives can improve their physical and chemical properties.
Depending on the carbon concentration, carbon compounds are divided into the following types:
- low-carbon (carbon content does not exceed 0.29%);
- medium carbon (up to 0.6%);
- high carbon (more than 0.6%).
Carbon compounds are used in various industrial sectors. Depending on the scope of application, alloying components are added to them to achieve specific properties, including heat resistance, corrosion resistance, etc. According to these criteria, they are divided into the following categories:
- structural;
- instrumental.
Manganese is added to tool tools, which significantly improves the quality of the metal. The melting point of carbon steel is 1535°C.
Features of Alloy Steel
Additional components are introduced into the composition of alloyed compounds. In certain quantities they give them the required properties. Depending on the concentration of such elements, they are divided into the following types:
- low-alloyed (with a concentration of 2.5%);
- medium alloyed (up to 10%);
- highly alloyed (over 10%).
By adding additional components, it is possible to increase strength, corrosion resistance and improve other characteristics. The alloying components are chromium, copper, nickel, nitrogen, vanadium, etc. The melting point of alloy steel ranges from 1400°C to 1480°C.
physical characteristics
Weight
The weight of the material varies depending on the amount of fixed carbon and the presence of a certain percentage of porosity. The specific gravity of cast iron at the melting point can be significantly reduced depending on the presence of impurities in the cast iron.
In addition, the linear expansion of the metal and the structure of cast iron changes depending on the state of each indicator. That is, these are dependent quantities.
The specific gravity of each cast iron differs depending on the type of material. Gray cast iron has a specific gravity of 7.1±0.2 g/cm3, white cast iron has a specific gravity of 7.5±0.2 g/cm3, and malleable cast iron has a specific gravity of 7.3±0.2 g/cm3.
The video below will tell you about some of the physical properties of cast iron:
https://youtube.com/watch?v=zGVW6Hqy0pc
Volume
The volume of cast iron, passing through the temperature of phase transformations, reaches an increase of 30%. However, when heated to 500ºC, the volume increases by 3%. Growth is aided by graphite-forming elements. Carbide-forming components inhibit volume growth. The same growth is prevented by applying galvanic coatings to the surface.
The carbon content is usually at least 2.14%. Thanks to the carbon content, cast iron has excellent hardness. However, the plasticity and malleability of the material suffers against this background.
We will talk about the density of cast iron below.
Density
The density of the described material, cast iron, is 7.2 g/cm3. If we compare other metals and alloys with cast iron, this density value is quite high.
Due to its good density, cast iron is widely used for casting various parts in industry. In terms of this property, cast iron is only slightly inferior to some steels.
Stainless steel
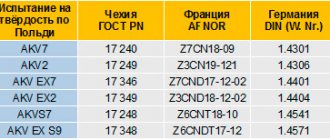
Stainless steel is one of the many iron alloys found in steel. It contains Chromium from 15 to 30%, which makes it rust-resistant, creating a protective layer of oxide on the surface, and carbon. The most popular brands of this type are foreign. These are the 300th and 400th series. They are distinguished by their strength, resistance to adverse conditions and ductility. The 200 series is of lower quality, but cheaper. This is a beneficial factor for the manufacturer. Its composition was first noticed in 1913 by Harry Brearley, who conducted many different experiments on steel.
At the moment, stainless steel is divided into three groups:
- Heat-resistant - at high temperatures it has high mechanical strength and stability. The parts that are made from it are used in the pharmaceutical, rocketry, and textile industries.
- Rust-resistant - has great resistance to rusting processes. It is used in household and medical devices, as well as in mechanical engineering for the manufacture of parts.
- Heat-resistant - resistant to corrosion at high temperatures, suitable for use in chemical plants.
The melting point of stainless steel varies depending on its grade and the number of alloys from approximately 1300 °C to 1400 °C.
What is the boiling point of iron
Melting temperature
chemically pure iron is 1539 o C. Technically pure iron, obtained as a result of oxidative refining, contains a certain amount of oxygen dissolved in the metal. For this reason, its melting point drops to 1530 o C.
The melting point of steel is always lower than the melting point of iron due to the presence of impurities in it. Metals dissolved in iron (Mn, Cr, Ni. Co, Mo, V, etc.) lower the melting point of the metal by 1 - 3 o C per 1% of the introduced element, and elements from the group of metalloids (C, O, S, P and etc.) at 30 – 80 o C.
Over most of the total duration of the melt, the melting temperature of the metal changes mainly as a result of changes in carbon content. At a carbon concentration of 0.1 - 1.2%, which is typical for finishing smelting in steel-smelting units, the melting temperature of the metal can be estimated with sufficient accuracy for practical purposes from the equation
Heat of fusion of iron
is 15200 J/mol or 271.7 kJ/kg.
Boiling point of iron
in recent publications it is given as 2735 o C. However, research results have been published according to which the boiling point of iron is much higher (up to 3230 o C).
Heat of vaporization of iron
is 352.5 kJ/mol or 6300 kJ/kg.
Saturated vapor pressure of iron
(PFe, Pa) can be estimated using the equation
where T is the metal temperature, K.
The results of calculating the saturated vapor pressure of iron at various temperatures, as well as the dust content in the oxidizing gas phase above the metal ( X
, g/m 3 ) are presented in Table 1.1.
Table 1.1
– Saturated vapor pressure of iron and dust content of gases at different temperatures
According to existing sanitary standards, the dust content in gases emitted into the atmosphere should not exceed 0.1 g/m3. From the data in Table 1.1 it is clear that at 1600 o C the dust content of gases above the open surface of the metal is higher than the permissible values. Therefore, it is necessary to purify gases from dust, consisting mainly of iron oxides.
Dynamic viscosity
. The coefficient of dynamic viscosity of the liquid () is determined from the relation
where F is the force of interaction between two moving layers, N;
S – contact area of layers, m2;
– velocity gradient of liquid layers normal to the direction of flow, s -1.
The dynamic viscosity of iron alloys usually varies within the range of 0.001 – 0.005 Pa•s. Its value depends on temperature and the content of impurities, mainly carbon. When the metal is overheated above the melting point above 25 - 30 o C, the influence of temperature is not significant.
Kinematic viscosity
fluid is the rate of momentum transfer in a unit mass flow. Its value is determined from the equation
where is the density of the liquid, kg/m3.
The value of dynamic viscosity of liquid iron is close to 6•10 -7 m 2 /s.
Iron Density
at 1550 - 1650 o C is equal to 6700 - 6800 kg/m 3. At the crystallization temperature, the density of the liquid metal is close to 6850 kg/m3. The density of solid iron at the crystallization temperature is 7450 kg/m3, at room temperature - 7800 kg/m3.
Of the common impurities, carbon and silicon have the greatest influence on the density of iron melts, reducing it. Therefore, liquid cast iron of ordinary composition has a density of 6200 - 6400 kg/m 3, solid at room temperature - 7000 - 7200 kg/m 3.
Calculation principle
Previously, Lindemann's formula was used to calculate the melting point of a metal. However, due to the low accuracy of the final calculations, it did not gain much popularity among foundries. In 1999, I.V. Gavrilin proposed a new system for calculating boiling and melting points:
Tm = DHm / 1.5 N0 k,
Explanation:
- Tmelting temperature.
- DHmelt - indicates the latent melting point.
- N0 is the designation of the latent heat of melting.
- k — Designation of Boltzmann's constant.
Transformation of ferrite grains into austenite grains
When ferritic iron is heated to a temperature of 912 ° C, the old composition of ferrite grains changes into a new composition of grains, already austenitic - a transformation occurs in the iron.
Imagine that the ferrite grain structure has just reached its transformation temperature. First we see the formation of new, very fine austenite grains, which are superimposed on the old ferrite grain boundaries. Then these grains grow until all the old ferrite grains disappear.
When ferrite transforms into austenite, two important phenomena occur:
1) Just like the transformation of ice into water, the transformation of iron from ferrite to austenite requires thermal energy. Therefore, when heated, the temperature of the iron will remain at about 912 °C until all the ferrite grains turn into austenite grains.
2) When ferrite transforms into austenite, volumetric changes occur. The density of austenite is 2% higher than that of ferrite, which means that an austenite atom occupies less volume than a ferrite atom.
All transformations in iron that occur when it is heated are shown schematically in Figure 3.
Figure 3
Concept of temperature scale
Some non-metallic objects also have similar properties. The most common is water. A temperature scale was developed regarding the properties of the liquid that occupies a dominant position on Earth. The reference points are the temperature of changes in the aggregative states of water:
- Transformations from liquid to solid and vice versa are taken to be zero degrees.
- Boiling (vapor formation inside a liquid) at normal atmospheric pressure (760 mm Hg) is taken to be 100 ⁰C.
Methods of obtaining
1. Iron (III) hydroxide can be obtained by reacting an ammonia solution with iron (III) salts.
For example, iron(III) chloride reacts with an aqueous solution of ammonia to form iron(III) hydroxide and ammonium chloride:
FeCl3 + 3NH3 + 3H2O = Fe(OH)3 + 3NH4Cl
2. Oxidation of iron (II) hydroxide with oxygen or hydrogen peroxide
:
4Fe(OH)2 + O2 + 2H2O → 4Fe(OH)3↓
2Fe(OH)2 + H2O2 → 2Fe(OH)3
3. Iron (III) hydroxide can be obtained by the action of alkali on a solution of iron (III) salt.
For example, iron(III) chloride reacts with a solution of potassium hydroxide to form iron(III) hydroxide and potassium chloride:
FeCl3 + 3KOH → Fe(OH)3↓ + 3KCl
A video of the production of iron (III) hydroxide by the reaction of iron (III) chloride and potassium hydroxide can be viewed here.
4. Iron (III) hydroxide is also formed by the interaction of soluble iron (III) salts with solutions of carbonates and sulfites. Iron(III) carbonates and sulfites are irreversibly hydrolyzed in aqueous solution.
For example: iron (III) bromide reacts with sodium carbonate. In this case, a precipitate of iron (III) hydroxide precipitates, carbon dioxide is released and sodium bromide is formed:
2FeBr3 + 3Na2CO3 + 3H2O = 2Fe(OH)3↓ + CO2 + 6NaBr
Iron(III) chloride reacts with sodium sulfite to form aluminum hydroxide, sulfur dioxide and sodium chloride:
2FeCl3 + 3Na2SO3 + 6H2O = 2Fe(OH)3 + 3SO2 + 6NaCl
Chemical properties
1. Iron (III) hydroxide exhibits weak amphoteric properties, with a predominance of basic ones. As a base, iron(III) hydroxide reacts with soluble acids.
For example, iron(III) hydroxide reacts with nitric acid to form iron(III) nitrate
:
Fe(OH)3 + 3HNO3 → Fe(NO3)3 + 3H2O
Fe(OH)3 + 3HCl → FeCl3 + 3H2O
2Fe(OH)3 + 3H2SO4 → Fe2(SO4)3 + 6H2O
Fe(OH)3 + 3HBr → FeBr3 + 3H2O
2. Iron (III) hydroxide reacts with acidic oxides of strong acids.
For example, iron(III) hydroxide reacts with sulfur(VI) oxide to form iron(III) sulfate
:
2Fe(OH)3 + 3SO3 → Fe2(SO4)3 + 3H2O
3. Iron (III) hydroxide reacts with soluble bases (alkalis). In this case, salts—ferrites—are formed in the melt, but the reaction practically does not occur in the solution. In this case, iron (III) hydroxide exhibits acidic properties.
For example, iron(III) hydroxide reacts with potassium hydroxide
in the melt with the formation of potassium ferrite and water:
KOH + Fe(OH)3 → KFeO2 + 2H2O
4. Iron (III) hydroxide decomposes when heated:
2Fe(OH)3 → Fe2O3 + 3H2O
A video experiment of the interaction of iron (III) hydroxide with hydrochloric acid can be viewed here.
This is interesting: Heat treatment of metals and alloys: we present it in detail
Melting temperatures of steel
Under certain conditions, solids melt, that is, they turn into a liquid state. Each substance does this at a certain temperature.
- Melting is the process of transition of a substance from a solid to a liquid state.
- Melting point is the temperature at which a crystalline solid melts into a liquid state. Denoted by t.
Physicists use a specific table of melting and crystallization, which is given below:
| Substance | t,°C | Substance | t,°C | Substance | t,°C |
| Aluminum | 660 | Copper | 1087 | Alcohol | — 115 |
| Voden | — 256 | Naphthalene | 80 | Cast iron | 1200 |
| Tungsten | 3387 | Tin | 232 | Steel | 1400 |
| Iron | 1535 | Paraffin | 55 | Titanium | 1660 |
| Gold | 1065 | Mercury | — 39 | Zinc | 420 |
Based on the table, we can safely say that the melting point of steel is 1400 °C.
This is interesting: Properties of steel - specific gravity, density kg cm3 and others
Briefly about the process
Melting aluminum at home is not such a difficult process as it may seem at first. The pieces of metal are heated to the required aluminum melting temperature in a special container.
The resulting melt must be kept in a heated state for some time and the resulting slag must be periodically removed from its surface. After this, pure liquid metal is poured into a special mold, in which it will cool for some time.
The time it takes to melt depends on the furnace itself, or more precisely on the temperature it can provide. If a gas burner is used instead of a furnace, then it should heat the metal from above.
https://youtube.com/watch?v=cIlonSuReH0