When welding, the parts are exposed to high temperatures, so it is very important to attach importance to the melting temperature of metals, taking it into account during the work process, since these indicators play an important role in the current parameters. In the burner, when gas is burned during the action of an electric arc, thermal power is created in order to destroy the crystal lattice of the metal. The melting characteristics of metals are given attention when selecting materials for the construction of units subject to frictional forces or metal structures that are exposed to temperatures.
What is melting point?
To find out at what temperature the metal melts, in laboratory conditions, the starting point at the beginning of the melting process is calculated to the hundredth of a degree. Moreover, this indicator does not depend on the force applied to the part.
When a certain pressure is created under vacuum conditions, metal blanks have the same melting point. This phenomenon can be explained by the accumulation of energy inside the substance, during which the bonds between molecules are destroyed.
How the process works
Elements, whatever they are: gold, iron, cast iron, steel or any other, melt approximately the same. This occurs due to external or internal heating. External heating is carried out in a thermal furnace. For internal applications, resistive heating is used, passing an electric current or induction heating in a high-frequency electromagnetic field . The impact is approximately the same.
When heating occurs , the amplitude of thermal vibrations of molecules increases. Structural lattice defects appear , accompanied by the rupture of interatomic bonds. The period of lattice destruction and accumulation of defects is called melting.
Depending on the degree at which metals melt, they are divided into:
- low-melting - up to 600 °C: lead, zinc, tin;
- medium-melting - from 600 °C to 1600 °C: gold, copper, aluminum, cast iron, iron and most of all elements and compounds;
- refractory - from 1600 °C: chromium, tungsten, molybdenum, titanium.
Depending on what the maximum degree is, the melting apparatus is selected. It should be stronger the stronger the heating.
The second important value is the boiling degree. This is the parameter at which liquids begin to boil. As a rule, it is twice the melting point. These values are directly proportional to each other and are usually given at normal pressure.
If the pressure increases, the amount of melting also increases. If the pressure decreases, then it decreases.
Difference between melting and boiling points
The melting point of metals is the point at which a solid crystalline substance transitions into a liquid state. In the composition of the melt, the molecules do not have their own location; they are held due to the force of attraction, therefore, in a liquefied state, the volume is retained, but the shape is lost.
During the boiling process, a loss of molecular volume occurs, and the molecules interact sluggishly with each other, moving chaotically in different directions, lagging behind the surface. Boiling point is the process by which the pressure level of metal vapor is balanced with the pressure of the external environment.
Carbon Steel Range
According to the degree of deoxidation, i.e., the content of oxygen dissolved in the metal, carbon steel can be boiling, calm, or semi-calm.
Boiling steel is not completely deoxidized and when the ingot solidifies, the carbon oxidation reaction continues to occur with the release of CO bubbles. The carbon content in this steel ranges from 0.02 to 0.27%.
Quiet steel is deoxidized in such a way as to eliminate the interaction of carbon and oxygen during the crystallization of the ingot. When smelting still steel in the main steelmaking units, it is deoxidized with manganese, silicon and aluminum.
Based on their chemical composition, mild steel is divided into carbon and alloy steel. Carbon steel is in turn divided into low- (09.29.2016
Melting point of various metals
According to knowledge from the section of physics, the process of transforming a solid into a liquid occurs only in bodies with a crystal lattice. The melting point of metals and alloys occurs in a different range of values. But it is very problematic to accurately calculate the boundary temperature of phase states in alloys. For pure elements, every degree is significant if these are compounds with slight fusibility.
Iron
The melting point of iron compounds must be high. If the element is technically pure, it melts at a temperature of 1,539 °C. Its substance contains sulfur inclusions, so its extraction requires a liquid state. Purified iron is also obtained through the electrolysis of metal salts.
Cast iron
Cast iron is considered the best material for melting. It has good fluid flow and shrinkage properties, so it can be used effectively in the casting process. Below are the boiling temperature indicators for cast iron:
A gray type of cast iron whose temperature reaches 1,260 °C. And when poured into molds, it increases to 1,400 °C.
A white variety of cast iron whose temperature rises to 1,350 °C.
One of the important points is that the temperature of cast iron is 400 units less than the same steel. Therefore, the processing of this material is less energy intensive.
Steel, melting point
The average melting point of steel is 1400 °C.
Steel is an iron-containing alloy containing carbon. Its main characteristic is strength. This is achieved due to the fact that it retains the parameters of volume and shape for a long time. In this case, the arrangement of molecules in the substance is in a balanced state. That is why a balance is achieved between the force of attraction and the force of repulsion.
The melting range of steel is higher than that of cast iron, so it is more energy-consuming.
Stainless steel
The melting point of stainless steel falls in the middle range between cast iron and steel. Stainless steel is a substance made of alloy steel that has anti-corrosion properties due to the chromium content of 11% or more in its composition.
The melting point of stainless steel ranges from 1,300 to 15,000 °C.
Aluminum and copper
The melting point of aluminum is 6,600 °C, so it has established itself as one of the medium-melting metals. Melting of pure copper compounds occurs at a temperature of 10,830 °C, and of alloys - 930 - 11,400 °C.
Silver and gold
Silver in its pure form melts at a temperature of 9,620 °C. Moreover, at the melting point of silver, it can be compared with the melting point in degrees of copper alloys.
Gold melts at a temperature of 10,640 °C.
Mercury
Mercury has the lowest melting point with a negative value. It is - 38.80 °C.
Iron and its properties
Iron is a chemical element that is number 26 on the periodic table. It is one of the most abundant elements in the entire solar system. According to research materials, the Earth's core contains approximately 79−85% of this substance . There is also a large amount of it in the earth's crust, but it is inferior to aluminum.
In its pure form, the metal is white with a slightly silvery tint. It is plastic, but the impurities present in it can determine its physical properties. Reacts to a magnet.
Iron is present in water. In river waters its concentration is approximately 2 mg/l of metal. In sea water its content can be a hundred or even a thousand times lower.
Iron oxide is the main form that is mined and found in nature. Iron oxide can be located in the uppermost part of the earth's crust and be a component of sedimentary formations.
An element in twenty-sixth place on the periodic table can have several oxidation states. It is they who determine its geochemical feature of being in a certain environment. In the Earth's core, the metal is present in a neutral form.
Melting point table
| Low-melting metals | |
| Lithium | + 180 °C |
| Potassium | + 63.60 °C |
| Indium | + 156.60 °C |
| Tin | + 2 320 °C |
| Thallium | + 3,040 °C |
| Cadmium | + 3 210 °C |
| Lead | + 3 270 °C |
| Zinc | + 4 200 °C |
| Medium melting metals | |
| Magnesium | + 6 500 °C |
| Aluminum | + 6 600 °C |
| Barium | + 7 270 °C |
| Silver | + 9 600 °C |
| Gold | +10 630 °C |
| Manganese | + 12 460 °C |
| Copper | + 10 830 °C |
| Nickel | + 14 550 °C |
| Cobalt | + 14 950 °C |
| Iron | + 15 390 °C |
| Duralei | + 6 500 °C |
| Brass | + 950 – 10 500 °C |
| Cast iron | + 1,100 – 13,000 °C |
| Refractory metals | |
| Titanium | + 16 800 °C |
| Platinum | + 17 690 °C |
| Chromium | + 19 070 °C |
| Zirconium | + 18 550 °C |
| Vanadium | + 19 100 °C |
| Iridium | + 24 470 °C |
| Molybdenum | + 26 230 °C |
| Tantalum | + 30 170 °C |
| Tungsten | + 34 200 °C |
PHYSICAL PROPERTIES
| Mineral color | iron black |
| Stroke color | grey |
| Transparency | opaque |
| Shine | metal |
| Cleavage | imperfect by {001} |
| Hardness (Mohs scale) | 4,5 |
| Kink | hackly |
| Strength | malleable |
| Density (measured) | 7.3 – 7.87 g/cm3 |
| Radioactivity (GRapi) | 0 |
| Magnetism | ferromagnet |
What does the melting temperature depend on?
Different materials also have different melting points, at which a radical restructuring of the lattice occurs to the state of liquid. Metal products and alloy products have the following features:
- Different materials also have different melting points, at which a radical restructuring of the lattice occurs to the state of liquid. Metal products and alloy products have the following features:
- They are rarely found in their natural form, i.e. without impurities. It is the composition that determines what the melting temperature should be. An example is tin, to which silver inclusions are added. Thanks to impurities, the material begins to become resistant to temperature.
- There are alloys that, due to their chemical composition, transform into a liquid state when the thermometer rises just above + 1,500 °C. There are also alloys that “stick” if they are heated to 30,000 °C.
- It is worth considering the fact that one of the most important properties of substances is their melting point. An example is aviation technology.
How the process works
Elements, whatever they are: gold, iron, cast iron, steel or any other, melt approximately the same. This occurs due to external or internal heating. External heating is carried out in a thermal furnace. For internal purposes, resistive heating is used, passing electric current or induction heating in a high-frequency electromagnetic field
. The impact is approximately the same.
When does heating occur
, the amplitude of thermal vibrations of molecules increases.
Structural lattice defects
appear , accompanied by the rupture of interatomic bonds. The period of lattice destruction and accumulation of defects is called melting.
Depending on the degree at which metals melt, they are divided into:
- low-melting - up to 600 °C: lead, zinc, tin;
- medium-melting – from 600 °C to 1600 °C: gold, copper, aluminum, cast iron, iron and most of all elements and compounds;
- refractory – from 1600 °C: chromium, tungsten, molybdenum, titanium.
Depending on what the maximum degree is, the melting apparatus is selected. It should be stronger the stronger the heating.
The second important value is the boiling degree. This is the parameter at which liquids begin to boil. As a rule, it is twice the melting point. These values are directly proportional to each other and are usually given at normal pressure.
If the pressure increases, the amount of melting also increases. If the pressure decreases, then it decreases.
Types of metal alloys
The types of metal alloys vary based on their melting point, so the alloy variants are as follows:
- Low-melting (tin, zinc, lead, bismuth) with a melting point of no more than 600 °C.
- Medium melting (aluminum, magnesium, nickel, iron) with a temperature of 600 - 1,600 °C.
- Refractory (molybdenum, tungsten, titanium) with a temperature of more than 1,600 °C.
Next, we’ll tell you a little about the types of steels, wood alloy and solders.
Features of Carbon Steel
This material contains an admixture of carbon, approximately 2.13%. Moreover, it is devoid of alloying additives, but contains impurities of silicon, manganese and magnesium.
Features of Alloy Steel
In addition to the carbon and iron content, additional elements are added to it to improve its properties.
Features of stainless steel
Stainless steel differs from carbon steel due to the content of the element chromium in its composition, due to the properties of which it is not susceptible to oxidation, and, therefore, to rust.
Features of tool steel
It also has a carbon composition (0.8 - 0.9%). Demonstrates hardness, strength, and is easy to process. Used in the manufacture of instruments, such as medical ones.
Wood's alloy
It is a material used in soldering parts for radio receivers, as well as in electroplating, when working in laboratory conditions with pesticides.
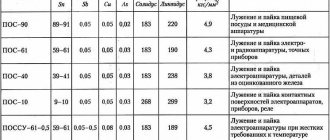
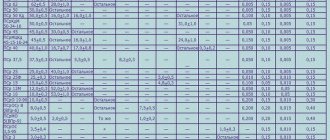
Soldering alloys
Another name for them is solders. Materials for solders are different. It all depends on what is included in the materials that need to be combined. For example, aluminum requires one alloy of solder, but copper is completely different.
Bibliography
- Kawakami K., Kitagawa T., Miyashita Y. et al. II Nippon Kokan Technical Report.Overseas. 1982. V. 36. P. 26...27.
- SchreweH. II Verlag Stakleisen mbH. Dusseldorf, 1987. S. 104.
- Deuxieme Conference Mondial des Founders a models perdus. Dusseldorf, 1…4 June, 1960.
- AymardJ. P., DetrezP. IIFouderie 330. Janvier, 1974. P. 11...24.
- Hirai M., Kanamru K., Mori H. IITetsu to Hagane 52 (1969). P. 85.
- Roeser Wm. R, Wensel H. T. Freezing Temperatures of High-Purity Iron and Some Steels // Journal of Research of the National Bureau of Standards. 1941. V. 26. P. 273…287.
- Kagava A., Okamota T. Influence of alloying elements on temperature and composition for peritectic reaction in plain carbon steel // Material science and technology. October 1986. V. 2. No. 10. P. 997...1008.
- Andrews KW Solidification ranges of steel // A note submitted to the alloy phase diagram date Committee of the Metals Society, 1981. P. 1…8.
- WolfM. //Zurich, 1982. S. 37...49.
- Howe AA II Ironmaking and Steelmaking. 1988. V. 16. No. 3. P. 134…142.
- Jerkontoret.//Stockholm, 1977. P. 117.
- Schiirmann E., Schweinichen JV, Volker R. ua II Giesserei-Forschung 39, Jahrgang 1987. H. 4. S. 133…136.
- SugdenA. A. V., Bhadeshia HKDH II Material science and technology. October 1989. V. 5. No. 10. P. 977…984.
You can ask a question you are interested in or write a comment on this article here. Write to us and we will definitely answer.
Melting aluminum cans at home
Today we will look at a way to melt aluminum cans using a simple small melting furnace at home. This time we're using our high-tech backyard and a bucket of soda or beer cans. To make crafts from aluminum, or rather from cans, let's start by taking out our mini-smelter, which was already made earlier, and a large bag of coal briquettes. They are commonly used for barbecues. When several coals are distributed at the bottom of the smelter, a crucible made from a steel fire extinguisher can be added.
Just look what they sell in this Chinese store.
If you place the crucible on a layer of coal, the cans will melt faster. Now we connect the steel tube through the air supply hole. This will provide enough temperature for melting, but we still need to find a way to get air inside. A household hair dryer, which can be bought at any store, is perfect for this.
Let's connect the hair dryer to a piece of PVC pipe, using two three-centimeter couplings to attach the steel tube on one side and make it easier to disconnect the hair dryer on the other. The entire structure is very easy to disassemble and place in a 20 liter bucket.
The blower is at the right angle, it doesn't hurt to prop it up so it doesn't move away. This way you will keep the walls intact and significantly increase the service life of the smelter. Now that the smelter is ready, let's fill it to the top with coal. You can use a propane torch because it heats everything up very quickly. The coals are burning, so let's turn the hairdryer on low and blast the oxygen into the coals to really heat things up. As you can see, the lid we made retains the heat and the temperature rises. The crucible and the holes in the center of the lid are precisely adjusted.
Now let's take aluminum cans prepared for melting and steel tongs. After 10 minutes the smelter is extremely hot. You can see that the steel crucible glows orange, which means everything is ready. The crucible is 8 centimeters in diameter and is therefore perfect for melting drink cans and at temperatures over 500 degrees Celsius they melt in just a couple of seconds. Let's bring the smelter's power to full to melt everything as quickly as possible. The productivity of the device is on average 10-12 cans per minute.
The nice thing is that the cans can be dirty and painted, with the remains of soda. No matter, as we will soon see, the mini-smelter absorbs everything and produces pure liquid aluminum. According to experience, 35-45 cans are enough to produce 450 grams of aluminum. If you crush the jars first, you don’t even have to remove the lid, which means that even less metal will oxidize during melting. After melting 50 cans, the crucible is full, but there is a lot of waste inside that we don’t need.
A good way to insulate aluminum is to use a steel form. To begin, carefully remove the crucible, making sure that you grip it very securely with steel tongs. Then very slowly pour the melt into a steel mold. As you can see, the slag remains in the shade or and acts almost like a filter, preventing solid particles from leaving it. Having separated what we need, we can tap the crucible on a piece of cement and remove the slag. Once the crucible is cleaned, we can use it again immediately.
For fun, a few more cans were melted down to fill a new muffin tin. The goal is to give the bars a beautiful, unusual appearance. The form is made of steel, but sometimes fire breaks out. This burns the non-stick coating. But this will happen only the first time. After a few minutes, the ingots begin to harden, but they are still terribly hot, so much so that the paper instantly catches fire from them. It's a good idea to have a bucket of water to cool them down. The ingots thrown into cold water are still hot enough to instantly boil it, but after about 10 seconds they cool down and can already be reached with your hands.
Use a mini muffin tin to make smaller ingots. The results were very cute little cupcakes. The purpose of ingots is to have pure metal ready when you want to do something. Now, if necessary, you just need to throw a couple of ingots into a clean crucible. With this configuration, the ingots will melt in 5-10 minutes. When using ingots, we do not need to get rid of slag, except perhaps a thin film of aluminum oxide, which means the crucible is full of liquid aluminum ready for casting.
Let's pour aluminum into sand, in which a special mold is made, which burns, absorbing 900 grams of liquid metal. After 10 minutes, the metal is hard enough to grip with pliers. We can break the mold and pull out our castings. At the link at the beginning of the article you can see how the sword was cast in more detail.
Shredding aluminum can scrap
Typically, aluminum cans are sent for remelting in the form of briquettes weighing up to 400 kg and with a density of no more than 500 kg/m3. These briquettes are convenient for transportation, but are not suitable for direct loading into an oven for melting into materials that will be used to make new cans. Therefore, these briquettes are crushed and sorted to ensure that they do not contain liquids or explosive materials. This is very important to ensure the safety of foundry workers and the safety of foundry equipment. For this purpose, a special grinding machine is used - a shredder.
Production of aluminum cans
Aluminum strips for can bodies and lids are supplied to can manufacturers. As a result of the aluminum can production process, about 20% of the aluminum strip (or 13% of the original melt) is returned to the ingot manufacturer in the form of production waste - the remains of sheets with holes in place of the cut blanks for can bodies and lids. In general, about 55% of the amount of the initial melt in the mixer goes into internal, production scrap. If all cans were returned as aluminum scrap, then to close the cycle of recycling old aluminum cans into new ones, only aluminum losses from waste would need to be replenished—just a few percent.
Processing steel to obtain special properties
To give a material certain properties or change them, alloying elements and various types of processing are used.
Some metals act as alloying elements. They can be chromium, aluminum, nickel, molybdenum
and others. In this way, certain electrical, magnetic or mechanical properties, as well as corrosion resistance, are achieved. Thus, stainless steel is obtained if it has been alloyed with chromium.
The properties of steel change by processing:
- thermomechanical (forging, rolling);
- thermal (annealing, hardening);
- chemical-thermal (nitriding, cementation).
Heat treatment is based on the property of polymorphism - when heated and cooled, the crystal lattice is capable of changing its structure. This property is characteristic of the basis of steel - iron, and therefore is inherent in it.
Different types of elements that may be present in steel
Carbon
. As the percentage of this element in steel increases, its strength and hardness increases. But there are losses in plasticity.
Sulfur
. This impurity is harmful because, together with iron, it forms iron sulfide. Because of it, cracks appear in the material as a result of the loss of bonds between grains during processing at high temperatures and under pressure. The presence of sulfur also negatively affects the strength of steel, its ductility, wear resistance, and corrosion resistance.
Ferrite
. This is iron, which has a body-centered crystal lattice. It is characteristic that alloys containing it are soft and have a plastic microstructure.
Phosphorus
. If sulfur reduces strength at high temperatures, phosphorus makes steel brittle at low temperatures. Nevertheless, there is a group of steels in which the content of this seemingly harmful element is increased. Products made from this metal are very easy to cut.
Cementite
, aka iron carbide. Its effect is opposite to that of ferrite. The steel becomes hard and brittle.