The alloying process is a technology for introducing particles of other metals into molten metal to form a uniform texture of the alloy and improve its qualities.
For the first time, targeted alloying was thought of in the second half of the 19th century: in 1858, the Frenchman Muschette came up with a steel for machine tools, to which manganese, carbon and tungsten were added. And steel with inclusions of carbon and manganese, invented in 1882 by the Englishman Robert Abbott Hadfield, went into mass production.
Alloying steels with chromium
More than 60% of the total global consumption of industrial chromium is used for the needs of ferrous metallurgy, where, due to its relative cheapness and ease of production, it is used as one of the main elements for alloying steels and cast irons. At the same time, steels alloyed with chromium, while maintaining basic performance characteristics, additionally acquire the beneficial properties inherent in Cr in the form of high indicators:
- hardness;
- corrosion resistance;
- heat resistance.
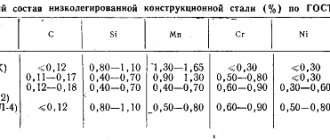
Alloyed steels are steels that contain, in addition to C and other common impurities, additions of a certain amount of alloying metals (Cr, Ni, Mo, etc.), as well as Mn and Si in dosages of 0.83...1.22%.
According to the volumetric content of the alloying composition, such steels are divided into three large groups:
- low-alloyed (total amount of alloying component ≤ 2.51%);
- alloyed (2.51...10.2%);
- highly alloyed (> 10%).
In turn, based on the intended use, alloy steels can be:
- structural;
- instrumental;
- belong to the category of special purpose steels.
AISI marking of stainless steels
| steel grade | Characteristics of stainless steel | Chemical composition, % |
| AISI 201, AISI 202 (analogs: 12X15G9ND, 12X17G9AN4) | They belong to the austenitic group of alloys. They contain chromium, nickel, manganese, copper and nitrogen. They provide a high level of strength to the finished product, are perfectly deformable and change their shape, and are characterized by high anti-corrosion properties. They are used in the manufacture of household appliances, pipelines and building structures. | |
| AISI 301 (analogue: 15Х17Н7) | Refers to non-magnetic steels. It has a high level of strength and ductility. Most often, such stainless steel is used in the manufacture of parts for automobiles and railway transport, household equipment and medical equipment. | Cr from 16 to 18; Ni from 6 to 8; Mn 2; Si 1; C 0.15; N 0.1; P 0.045; S 0.03 |
| AISI 302 (analog: 12Х18Н9) | Characterized by high levels of strength and ductility. It is resistant to corrosion. | Cr 17-19; Ni 8-10; Mo 4-5; Si 2-3; Mn <2; C <0.15; N >0.1; P <0.045; S <0.03 |
| AISI 303 (analog: 12Х18Н9) | Refers to austenitic steels. Has a high sulfur content. This steel is non-magnetic and is optimally used in mechanical and moving components. | Cr 17-19; Ni 8-10; Mn <2; Si <1; P <0.2; S >0.15; C <0.15 |
| AISI 304 (analogue: 08Х18Н10) | This stainless steel is highly resistant to corrosion and can be used in aggressive environments. In addition to the food industry, it is used for pharmaceutical, petroleum, chemical and textile production. | Cr 18-20; Ni 8-10.5; Mn <2; C <0.08; P <0.045; S <0.03 |
| AISI 316 (analogues: 03Х17Н14М3 and 04Х17Н13М2) | The brand contains molybdenum, which increases the material’s resistance to corrosion. The alloy is resistant to elevated temperatures. | Cr 15-17; Ni 14-16; Mo 2.5-3; C up to 0.03 |
| AISI 316Ti (analog: 10Х17Н13М2Т) | This stainless steel is enriched with titanium, which makes the material resistant to chlorine. Titanium also makes the alloy more durable and resistant to high temperatures. | Cr 15-17; Ni 14-16; Mo 2.5-3; C up to 0.03; Ti 1 |
| AISI 321 (analogue: 08Х18Н10Т) | The alloy is characterized by a high titanium content, is easily amenable to any type of processing, and is resistant to elevated temperatures, up to 800 degrees. It is used in the production of seamless pipes, in the manufacture of seam products and boilers, as well as in the construction of furnace systems. Also used in mechanical engineering, shipbuilding, and aviation. | Cr 17-19; Ni 9-11; Mn <2; Ti <1; Si <0.8; Cu <0.3 |
| AISI 403 (analog: 15Х12) | This alloy belongs to the martensitic-ferritic group. It is characterized by increased ductility and resistance to high temperatures. Products made from such steel can withstand loads in a slightly aggressive environment. | Fe 86; Cr 12.3; Mn 1; Si 0.5; C 0.15; P 0.04; S 0.03 |
| AISI 409 (analogue: 08Х13) | It is distinguished by its high chromium content and the addition of nickel, which allows it to be amenable to all types of processing. This stainless steel is used to make parts that will be subject to shock loads during operation: valves of presses and pumps, household items. The alloy is also used in the manufacture of products exposed to mildly aggressive environments: steam turbine blades, valves, bolts and pipes. | Fe ≈84; Cr 12-14; Mn up to 0.8; Si up to 0.8; Ni up to 0.6; C up to 0.08; P up to 0.03; S up to 0.025 |
| AISI 410 (analog: 12Х13) | This steel is characterized by ductility and strength. It withstands high temperatures and does not corrode in aggressive environments. | Cr 13.5; Mn 1.0; Si 1.0; Ni 0.6; C 0.15; P 0.045; Si 0.03 |
| AISI 416 | Belongs to the group of martensitic stainless steel, from which rolled sheets, metal profiles, and pipeline products are made. | Cr 12-14; Mn 1.25; Si 1; Se >0.15; C 0.15; P 0.06; S 0.06 |
| AISI 420 (analog: 40Х13) | This alloy is characterized by an increased level of heat resistance, wear and corrosion resistance. | Fe ≈84; Cr 12-14; Si up to 0.6; Mn up to 0.6; Ni up to 0.6; C 0.35-0.44; P up to 0.03; S up to 0.025 |
| AISI 430 (analog: 12Х17) | The grade belongs to the group of ferritic steels and has a balanced composition and good performance characteristics. | Fe ≈81; Cr 16-18; C up to 0.12; Si up to 0.8; Mn up to 0.8; P up to 0.035; S up to 0.025 |
| AISI 439 (analogue: 08Х17Т) | Stainless steel belonging to the ferritic group. Used in mechanical engineering, food industry, architecture and construction. | Fe ≈79; Cr 16-18; Si up to 0.8; Mn up to 0.8; Ti up to 0.8; Ni up to 0.6; Cu up to 0.3; C up to 0.08; P up to 0.035; S up to 0.025 |
| AISI 441 | Belongs to the group of ferritic alloys with a large amount of chromium. | Cr 16-18; Si up to 0.8; Mn up to 0.8; Ti up to 0.8; Ni up to 0.6; Cu up to 0.3; C up to 0.08; P up to 0.035; S up to 0.025 |
Structural alloy steels
marked using numbers and alphabetic abbreviations (for example, 15Х, 10Г2СД, 20Х2Н4А, etc.). The two-digit digital combination at the beginning of the brand displays the average C content in hundredths of a percent. The capital letter of the Russian alphabet indicates the name of the alloying element, in particular: B – (Nb), N – (Ni), Ф – (V), В – (W), М – (Mo), Х – (Cr), Г – (Mn), P – (P), C – (Zr), D – (Cu), P – (B), Ch – rare earth, E – (Se), C – (Si), Yu – (Al), K – (Co), T – (Ti), A – (N) only in the middle of the designation.
Numerical values after the letter abbreviation indicate the percentage of the alloying element. If there are no numbers, this means that the concentration of the alloying element is ≤ 1.5%.
The main volume of alloyed structural steels is smelted in the high-quality category (for example, 30ХГС).
If the letter “A” is located at the end of the brand name, this means that this steel is classified as a high-quality alloy steel (for example, 30KhGSA).
The presence of the letter “A” in the middle of the grade (for example, 16G2AF) indicates that this steel was also alloyed with nitrogen.
The letter “Ш” after the dash at the end of the brand name indicates that it belongs to the category of especially high-quality alloy steels (for example, 30ХГС-Ш, 30ХГСА-Ш).
If the structural alloy steel is cast, the letter “L” is added at the end of the grade designation (for example, 15GL, 40HNL, etc.).
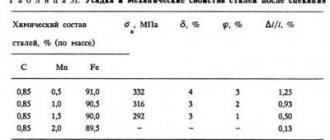
Structural alloy chromium steels (0.6...1.6% Cr) are characterized by increased strength, hardness and ductility combined with high cold resistance. The presence of chromium also contributes to a decrease in elongation. Thus, the tensile strength of ordinary steel 40 is 580 MPa, the yield strength is 340 MPa, and the elongation rate is 19%. In chromium steel grade 40X, the values of similar indicators change, respectively, to 1000 MPa, 800 MPa and 13%. Such steels are indispensable in the production of shafts, gears, pushers, worm gears, hardware and other industrial products.
Structural steels alloyed with chromium
Tool steels alloyed with Cr
In steel grades for this purpose (for example, 9ХФ, 7Х3, 3Х2В8Ф, etc.), the initial number indicates the average C content in tenths of a percent, provided that the steel contains 10 percent of it).
Depending on the predominance of the elements contained in the cast iron, alloyed cast irons are conventionally divided into classes:
- chromium;
- nickel;
- silicide;
- aluminum, etc.
Cast iron alloyed with chromium
Each of the alloying elements enriches cast iron with its own specific properties.
Cr, the main alloying element, performs ferrite-forming and carbide-forming functions, providing, in addition, high wear, corrosion and heat resistance of chromium cast iron.
Giving cast iron wear-resistant characteristics is ensured by the presence of a carbide strengthening phase in their structure. The criterion for determining the degree of wear resistance is the level of hardness provided by the carbide phase. The highest wear resistance is inherent in cast irons containing carbides (Cr, Fe, Mn)7C3, which are twice as hard as cementite-type carbides. The minimum amount of Cr contained in cast iron required for the formation of carbides (Cr, Fe, Mn)7C3 at 3% C content can vary in a fairly wide range (11...28%).
In sparingly alloyed cast irons (up to 2.5% Mn and 1.5% Ni) at 3% C, the Cr content required to obtain 100% carbides (Cr, Fe, Mn)7C3 should be >17%.
In order to impart high corrosion resistance to cast iron operated without additional heat treatment, it is necessary to introduce ≥ 22% Cr into its composition at 3% C content.
Ni in cast iron, being an austenite-forming element, helps to increase the characteristics of toughness, ductility and corrosion resistance.
Mn in the alloying composition performs mainly the function of a stabilizing element and catalyst, making the processes of carbide formation and austenitization more intense and smooth.
According to GOST 7769-82, the presence of certain special properties of cast iron and the percentage composition of chemical elements in the alloying composition are indicated by markings. For example, IChKh4G7D is a grade of wear-resistant cast iron alloyed with 4% Cr, 7% Mn and up to 1% Cu; ZhChKh2.5 – grade of heat-resistant cast iron alloyed with 2.5% Cu; ChH32 – grade of chromium cast iron containing up to 32% Cr; ChN19Х3Ш – grade of nickel heat-resistant cast iron, which contains 19% Ni, 3% Cr with nodular graphite, etc.
Alloyed cast irons with special properties, incl. chromium are a universal construction material used in many industries. They are widely used in the manufacture of machines and mechanisms operating under conditions of intense corrosion, abrasive and hydroabrasive wear for mining and ore dressing, metallurgy, energy, production of building materials and construction equipment, and other equipment for similar purposes.
phones: 8 (495) 366-00-24 (495) 504-95-54 (495) 642-41-95
Characteristics of high chromium content steel alloy
Cr (chromium) occurs in its natural state in most cases as iron chromite - Fe(CrO2)2. By reduction with carbon (coke) in electric furnaces, ferrochrome is produced, which is used in the rolling of alloy steels.
The more chromium in the alloy, the better it resists aggressive environments. Resistant to corrosion in salt and fresh water, alkaline and saline solutions, gas environments and petroleum products.
Chromium is responsible for the high anti-corrosion properties of the metal. In its pure form, the chemical element has increased stability due to the formation of a protective oxide film on the surface.
The presence of chromium in steel of more than 13% ensures the protective capabilities of rolled products only in a slightly aggressive environment. A threshold of 17% increases the properties of ferroalloys to resistance to more aggressive environments and oxidation processes.
Each production requires chrome steel with a different percentage of this chemical element. There are several main types of ferrosteel alloys.
The influence of chemical composition on the mechanical properties of steel
Each chemical element that makes up steel affects its mechanical properties in its own way - improves or worsens.
Carbon (C), which is an essential element and is usually found in steel in the form of the chemical compound Fe3C (iron carbide), increases its content to 1.2%, increases the hardness, strength and elasticity of steel and reduces toughness and weldability. This also reduces machinability and weldability.
Silicon (Si) is considered a beneficial impurity and is introduced as an active deoxidizer. As a rule, it is contained in steel in small quantities (up to 0.4%) and does not have a noticeable effect on its properties. But when the silicon content is more than 2%, the steel becomes brittle and breaks during forging.
Manganese (Mn) is contained in ordinary carbon steel in small quantities (0.3-0.8%) and does not have a serious effect on its properties. Manganese reduces the harmful effects of oxygen and sulfur, increases the hardness and strength of steel, its cutting properties, increases hardenability, but reduces resistance to impact loads.
Sulfur (S) and phosphorus (P) are harmful impurities. Their content, even in small quantities, has a harmful effect on the mechanical properties of steel. A steel content of more than 0.045% sulfur makes the steel red-brittle, i.e. one that cracks when forged in a heated state. Steel is protected from red brittleness by manganese, which binds sulfur into sulfides (MnS). The content of phosphorus in steel is more than 0.045%, making the steel cold-brittle, i.e. easily broken when cold. Phosphorus somewhat improves the machinability of steel, as it promotes chip separation.
How to determine the grade of steel: checking the type and composition of the metal
All over the world, certain standards have been adopted on how to mark metal products (rolled metal and others) before sale. Unfortunately, they are not international, but even knowledge of Russian GOST does not always help in understanding what kind of alloy lies in front of you. After all, the metal structure may not have quality marks, and may also be old. Then you will need knowledge of how to determine the grade of steel, as well as the type of metal depending on the composition, because there are many types of iron-containing alloys. We'll talk about this in our article.
First, let's tell you what we're dealing with. Steel sheets, bars and other workpieces are made from metal solid solutions containing iron and carbon as a base. Both substances are necessary because Fe without the addition of C is not a very hard metal. It gains hardness and wear resistance just after alloying. Depending on the carbon content, the first classification of steels is carried out into:
high-carbon – more than 0.6%;
medium carbon – up to 0.6%;
low carbon – less than 0.25%.
They all have different characteristics and are used in different situations.
Alloying with other materials – metals and non-metals – is actively used. Their inclusion makes it possible to achieve a more perfect composition, which acquires unique properties, for example, with a high concentration of chlorine, stainless steel is obtained, since the substance enters into a chemical reaction with oxygen and an oxide film is formed on the surface. So the following can be added: molybdenum, titanium, nickel, copper and other components. The marking, which is the main source of how to find out the grade of steel, lists the letters of those elements that are presented in preference. The content of smaller impurities is usually not taken into account in the name. Thus, alloying also carries two different classifications - this is by degree:
Highly alloyed - more than 10%.
Medium alloyed - up to 10%.
And according to the substance/s in the composition - chromium-nickel alloy, chromium, etc.
There are several different labeling regulations around the world. The most common are European, American and Russian. The latter, it would be correct to say Soviet, is more relevant for us and for the states that are part of the CIS. We will continue to concentrate more on this.
The influence of chemical elements on the properties of steel.
Catalog
Our Instagram
The influence of chem. elements on the properties of steel.
Symbols of chemical elements:
| chromium (Cr) - X nickel (Ni) - H molybdenum (Mo) - M titanium (Ti) - T copper (Cu) - D vanadium (V) - F tungsten (W) - B | nitrogen (N) - A aluminum (Al) - Yu beryllium (Be) - L boron (B) - P bismuth (Bi) - Vi gallium (Ga) - Gl | iridium (Ir) - And cadmium (Cd) - Kd cobalt (Co) - K silicon (Si) - C magnesium (Mg) - Ш manganese (Mn) - G | lead (Pb) - AC niobium (Nb) - B selenium (Se) - E carbon (C) - U phosphorus (P) - P zirconium (Zr) - C |
INFLUENCE OF IMPURITIES ON STEEL AND ITS PROPERTIES
Carbon - found in steel usually in the form of a chemical compound Fe3C called cementite. With an increase in carbon content to 1.2%, the hardness, strength and elasticity of steel increase, but ductility and impact resistance decrease, and workability deteriorates, and weldability also deteriorates.
Silicon - if it is contained in steel in small quantities, it does not have a special effect on its properties. (Useful impurity; introduced as an active deoxidizer and remains in the steel in an amount of 0.4%)
Manganese , like silicon, is found in ordinary carbon steel in small quantities and does not have any particular effect on its properties. (A useful impurity; introduced into steel for deoxidation and remains in it in an amount of 0.3-0.8%. Manganese reduces the harmful effects of oxygen and sulfur.
Sulfur is a harmful impurity. It is found in steel mainly in the form of FeS. This compound makes the steel brittle at high temperatures, such as during forging, a property called red brittleness. Sulfur increases the abrasion of steel, reduces fatigue resistance and reduces corrosion resistance. In carbon steel, no more than 0.06-0.07% sulfur is allowed. (Steel is protected from red brittleness by manganese, which binds sulfur into MnS sulfides).
Phosphorus is also a harmful impurity. Reduces viscosity at low temperatures, that is, it causes cold brittleness. Phosphorus somewhat improves the machinability of steel, as it promotes chip separation.
ALLOYING ELEMENTS AND THEIR INFLUENCE ON STEEL PROPERTIES
Chromium (X) is the cheapest and most common element. It increases hardness and strength, slightly reducing ductility, and increases corrosion resistance; the content of large amounts of chromium makes the steel stainless and ensures resistance to magnetic forces
.
Nickel (N) - imparts corrosion resistance
, high strength and ductility,
increases hardenability
, affects the change in the coefficient of thermal expansion. Nickel is an expensive metal; they are trying to replace it with a cheaper one.
Stainless steels: how composition affects properties
Alloy steels occupy a significant share of the metallurgical products market.
These include the so-called “stainless steels” - a group of alloys characterized by increased resistance to corrosion. Since their appearance, the range of such steels has expanded to several hundred items. Therefore, a system for their classification and labeling was developed. It is worth noting that the name “stainless steel” does not entirely correctly reflect its properties. Any iron-carbon alloy is exposed to oxygen and aggressive substances, but for this to affect its performance properties, it takes different times. Therefore, stainless steels are more correctly called corrosion-resistant.
By composition
Chromium, nickel, vanadium, molybdenum, titanium and some others are used as alloying additives that increase the resistance of the iron-carbon alloy to rust formation.
Corrosion resistance is also increased by manganese and silicon introduced to deoxidize and neutralize sulfur. Based on the main alloying elements, stainless steels are classified as chromium, manganese, etc.
Some additives are used to impart special structural or technological properties to steels, for example, to crush carbides and increase impact toughness.
The basic alloying elements of stainless steel are chromium and nickel. They both enter into solid solution with iron and increase corrosion resistance.
When oxidized, they form a thin oxygen-impermeable film on the surface of a steel product, resistant to chemical, electrochemical and atmospheric influences. Nickel expands the austenite region in iron-carbon alloys.
Chromium narrows it, but is a carbide-forming element and binds carbon. The ratio of nickel and chromium has a decisive influence on impact strength, weldability and ability to withstand cold deformation.
Carbon, as one of the essential components of steels, negatively affects corrosion resistance. However, the hardness and wear resistance of steel depends on its content. For example, 95Х18 has less pronounced corrosion-resistant properties compared to 40Х13, despite its higher chromium content.
By properties
A more clear idea of alloys is given by dividing them into groups according to properties:
- Corrosion resistant. Steels are highly resistant to atmospheric corrosion and can be used under normal conditions in a loaded state. Examples include stainless steel used for the manufacture of utensils and equipment for the food industry: 08Х18Н10, 20Х13, 30Х13.
- Heat resistant. A distinctive feature of such alloys is their high resistance to scale formation at high temperatures. Heat-resistant stainless steels are used for the manufacture of heat exchangers for boiler and pyrolysis plants (15Х28), valves for automobile and aircraft engines (40Х10С2М), parts for heating metallurgical furnaces (10Х23Н18).
- Heat resistant . A number of alloys have been developed that can operate under load at high temperatures without significant deformation and destruction. They use complex alloying systems (05Х27У5, 15Х12ВН14Ф, 37Х12Н8Г8МФБ). Steel type 20Х13 also has moderate heat resistance.
By structure
Based on their microstructure, stainless steels are divided into the following classes:
- austenitic;
- ferritic;
- martensitic;
In addition to them, there are intermediate groups:
- austenitic-ferritic;
- martensitic-ferritic;
- martensitic-carbide.
Heat treatment has a great influence on corrosion resistance, since it affects the phase composition of most stainless steels. Stability decreases when carbide heterogeneity occurs. This phenomenon is caused by the so-called intercrystalline corrosion.
When steels are heated to temperatures in the range of 500 – 800 °C, chains of carbides and areas with a reduced chromium content are formed at the grain boundaries. In the body of the grain, the content of alloying elements remains high. This type of corrosion is often observed in weld areas.
To combat this phenomenon, the steel composition is stabilized by introducing a small amount of titanium.
Austenitic steels
During crystallization, austenitic steels form a single-phase system with a face-centered crystal lattice. One of the most prominent representatives of the class is alloy 08Х18Н10.
Due to the high nickel content in stainless steels of this class (up to 30%), the austenite phase remains stable down to – 200 °C, and the carbon content does not exceed 0.12%. Steels with this structure are characterized by the absence of magnetic properties.
Most of them have good machinability.
Austenitic steels are necessarily subjected to heat treatment - hardening, tempering or annealing. The cooling rate practically does not change the hardness, but it affects the resistance to liquid and gaseous aggressive media, stabilizes the grain size and resistance to deformation.
Additional elements are introduced into the alloying systems of austenitic chromium-nickel steels:
- molybdenum – to prevent pitting and operation in reducing atmospheres
- titanium and niobium – for protection against intercrystalline corrosion.
- silicon – to increase acid resistance;
- manganese - to improve casting qualities.
Ferritic steels
This class includes chromium steels with low carbon content. They have a body-centered cubic lattice, which determines their magnetic properties.
Ferritic steels have lower corrosion resistance compared to austenitic steels and cannot be strengthened by heat treatment, but have higher technological properties. They are easier to machine and weld better, and their cost is significantly lower.
At temperatures of 300 – 400 °C, steels acquire high ductility, and they can be used to produce volumetric stamped parts of complex shapes.
chromium in such steels reaches 27%. Molybdenum, titanium and aluminum are used as stabilizing additives.
Martensitic steels
Alloys of this class contain at least 0.15% carbon and 11% chromium. Martensite has a microscopic needle-like structure and, when magnified, looks the same as carbon steel after hardening.
The crystal lattice has a tetragonal shape and is characterized by high internal stresses. This determines high strength properties and hardness. For example, for 40X13 it is up to 52 - 55 HRC.
Molybdenum, niobium, vanadium and tungsten are introduced as additional alloying elements. Martensitic steels, due to their high hardness, are difficult to cut and have low ductility.
One of the main technological properties of corrosion-resistant steels with this structure is the ability to self-harden. Martensitic transformation occurs upon cooling in air. To increase heat resistance, steel after hardening is tempered with sorbitol or troostite.
The influence of alloying elements on the properties of steel
gains strength after tempering at 550°; meanwhile, the impact resistance in the first case is 10, and in the second - 7 kgm/cm 2.
Thus, it should be considered that silicon in amounts up to about 1.5% has a rather positive effect on the properties of tempered steel; Silicon steels containing up to 1.5% Si, when processed to the same hardness as unalloyed ones, have a slightly higher reserve of toughness, and at the same tempering temperature they surpass unalloyed steel in terms of strength, but are inferior to it in terms of toughness. At the same time, the introduction of a significant amount of silicon (more than 2% Si) into the steel being improved is accompanied by a deterioration in its viscosity and temperature reserve of viscosity.
Manganese. In Fig. 191 shows the effect of manganese on the tensile strength and elongation of improved steel with different carbon content. The figure shows that with increasing manganese content in steel, the tensile strength increases slightly, and the relative elongation, on the contrary, decreases. It is typical that the lower the carbon content in the steel, the more noticeable the effect of manganese.
The influence of manganese on the general complex of mechanical properties of improved steel with the same carbon content is shown according to the author’s data in Table. 68. An increase in manganese content from 0.45 to 1.35% has a relatively weak effect on the mechanical properties of steel containing 0.25-0.28% C; at a higher manganese content (up to 2.79%), a significant increase in strength indicators is observed while simultaneously significantly reducing ductility and toughness.
The effect of manganese is more noticeable in the case of impact testing at subzero temperatures. In Fig. 192, according to the author's data, shows the effect of manganese on the impact strength of samples processed to a hardness of 228-217 Nb at different test temperatures. As can be seen from the data presented, an increase in manganese content from 0.45 to 1.35% causes a slight increase in the temperature reserve of viscosity, but even in this case, steel with 2.79% Mn showed a high tendency to brittle fracture.
The negative effect of increased amounts of manganese on the viscosity of thermally improved steel with 0.35-0.40% C was also established by V.D. Sadovsky and N.P. Chuprakova, who concluded that “only with a manganese content not exceeding 1 .5%, you can count on good impact strength.”
There are, however, indications that at a low carbon content in steel, the presence of significant amounts of manganese (up to 3-5%) does not cause a deterioration in the toughness of thermally improved steel.
In Fig. 193 shows the effect of manganese on the mechanical properties of steel with different carbon contents after quenching from 900° and high tempering at the same temperature. In the case of carbon content in the range of 0.09-0.14%, even at 4% MP, the impact strength invariably remains at a very high level, while the tensile strength and yield strength increase.
In steel with 0.25-0.37% C, an increase in manganese content above 3% is accompanied by a decrease in viscosity. I. E. Kontorovich believes that: “steels with a low carbon content (0.12-0.15%) and 3-5% manganese have high mechanical properties. A sharp decrease in toughness is found only in steels with a higher carbon content at the same manganese content.”
Some other authors hold a similar opinion.
Thus, in thermally treatable steels, the negative effect of large quantities of manganese is detected only in the presence of a significant amount of carbon, when
the lower the carbon content, the higher the manganese content in steel can be allowed. At least, with a content of up to 1.8-2.0% Mn, it is still impossible to state its harmful effect on medium-carbon structural steel
(0.2-0.4% C). This is also confirmed by extensive experience in the use of manganese steels in industry.
Chromium. The effect of chromium on the mechanical properties of steel after quenching and high tempering is shown in Table. 69. From the data in the table it is clear that in steel tempered at 600°, an increase in chromium content is accompanied by an increase in strength and some loss of toughness while maintaining ductility at approximately the same level. The influence of chromium weakens somewhat when steel is tempered at 650°. This is explained by the fact that chromium slows down the precipitation and coagulation of carbides during tempering, slightly increases the recrystallization temperature of the a-phase and therefore noticeably delays the softening of steel at 600°. However, the effect of its action is sharply weakened at 650°, since the tempering temperature region in this case turns out to be greatly shifted from those zones in which carbide formation develops (500-550°), as well as recrystallization (550-600°) of the os-phase in chromium steels on vacation.
Due to the fact that with increasing chromium content at the same tempering temperature, strength indicators increase, the true effect of chromium on impact strength is “disguised”. More reliable ideas about the effect of chromium on toughness can be obtained by comparing the properties under the condition of the same strength or hardness of steel.
In Fig. 194 shows the effect of chromium on the impact strength of steel with various carbon contents, processed to a tensile strength of 100 kg/mm 2. From the figure it is clear that when
Author:
Administration
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
Chrome steels
Chromium is a common and cheap alloying element, which is used for alloying both cast and powder steels in order to increase strength, wear resistance and give them special physical and chemical properties.
Chromium forms a continuous series of solid solutions with iron (Fig. 7). When up to 7% Cr is introduced into iron, points A4 and A3 on the state diagram of the Fe-Cr system decrease. With a further increase in chromium content, the point continues to decrease, and point A3 begins to increase, and at 12.8% Cr these points merge, i.e. the region of γ-iron solid solutions is closed. At 42-48% Cr in the iron-chromium alloy, an extremely brittle Fe-Cr intermetallic compound called the o-phase precipitates. In the state diagram of the Fe-Cr system, under the influence of carbon, the region of the y-solid solution expands significantly and closes not at 12.8% Cr, as in the double iron-chromium alloy, but at a higher chromium content. In iron-carbon alloys, under the influence of chromium, point A4 decreases sharply and at 12% Cr almost merges with point A3. Points E and S rise and simultaneously move to the left towards lower carbon content.
Chromium significantly increases the incubation period and the time of complete decomposition of austenite during its isothermal transformation. Chromium steel is less prone to overheating than simple carbon steel, since chromium carbides, going into solid solution at a higher temperature than cementite, prevent the growth of austenite grains.
Chromium increases the stability of austenite, increasing its tendency to overcool, and significantly reduces the critical hardening rate, improving the hardenability of steel.
Chromium is a strong carbide-forming element. In steel, chromium carbides are always double or complex: part of the chromium in them is replaced by iron or other elements. The higher the chromium content in steel, the larger part of it is included in complex carbides. For example, carbide (Fe-Cr)3C exists in pearlitic steel with a chromium content of up to 5%. Carbides Cr7C3 and Cr23C6 are formed in steel of the martensitic and carbide classes. Chromium increases strength and gives steel special properties, so it is used for alloying both cast and sintered steel of various classes and purposes.
The properties of powder chromium steels largely depend on the method of introducing chromium and the structure formed depending on this. The introduction of chromium in the form of pure powder leads to the formation of an extremely heterogeneous structure, which is due to the slowdown in the dissolution of chromium in the iron base due to its high ability to oxidize and carbide formation.
When sintering chromium steel obtained from a mechanical mixture of pure components, the formation of carbides begins in the temperature range of 900-950 °C due to the diffusion of carbon and iron to chromium particles. In place of undissolved chromium particles, carbides (Fe,Cr)3C and (Fe,Cr)23C6 are formed, having a hardness of 892-1430 HV. Around the carbides, a zone appears, enriched in carbon and representing a solid solution of chromium in iron with an increased concentration and having a high microhardness of 376-592 HV. Near the carbon-enriched zone, there are areas of the microstructure depleted in carbon, which then transform into the base, consisting of areas of chromium ferrite and the eutectoid Feа + (Fe,Cr)3C with microhardness 80-100 and 224-322 HV, respectively. During the holding process during sintering, some equalization of carbon and chromium concentrations occurs, but complete homogenization of the solid solution at sintering temperatures of 1150 - 1200 C does not occur, as a result of which the entire spectrum of structures is observed in sintered chromium steel - from ferritic to troostomartensitic. The hardness of the base ranges from 1.02 * 10v9 to 6.52 * 10v9 Pa. With an increase in the amount of chromium, the volume fraction of primary carbides increases, the average size of which is 18-22 microns.
The production of chromium steels from alloy powders ensures the formation of a more uniform structure. The mechanical properties of chromium steels obtained from alloyed powders and mechanical mixing of components are given in the work. The ductility of steels made from alloyed powders is three to four times higher than that of steels made from mixtures of powders (Fig. 8). Tensile strength is approximately at the same level for low-alloy steels and slightly higher for high-alloy steels made from alloy powders. Low ductility and reduced strength of high-alloy chromium steels obtained by mixing powders are associated with increased heterogeneity of the structure.
Reducing the heterogeneity of the structure can be achieved by increasing the dispersion of the initial powders or using less concentrated additives. In table Figure 8 shows the properties of an iron-chromium alloy obtained by mixing X30 steel powder of various dispersions with annealed carbonyl iron powder. With increasing dispersion of powders, the properties of steel increase.
At a temperature of 1000 °C, there is no noticeable dissolution of X30 particles in iron. The microhardness of the particles is 135 HV, the microhardness of the iron base is 92 HV. With an increase in temperature to 1100 °C, small high-chromium particles dissolve with the formation of a narrow rim of a solid solution of chromium in iron around them.
The dissolution of large X30 particles with the formation of a solid solution zone and a small number of diffusion pairs in the particles begins at a temperature of 1200 °C.
Sintering at a temperature of 1300 °C for 3 hours promotes more complete diffusion of chromium. The dissolution of large particles is accompanied by a decrease in their microhardness to 115 HV, enlargement of diffusion pores and the formation of continuous porous layers in the places of former X30 particles. Highly dispersed additives are completely dissolved. The small diffusion pores formed in this case are arranged in the form of thin broken chains.
With an increase in holding time to 4.5 hours, the material with the addition of large X30 particles acquires the structure of chromium-alloyed iron with areas of a dense network of diffusion porosity in the places where the particles were previously located. When alloying iron with highly dispersed additives X30, a small number of pores are formed and a homogeneous structure is obtained at a temperature of 1300 C and holding for 4.5 hours. In the case of large particles, the degree of homogeneity of the structure is much lower.
In the work, powdered chromium steel containing 2% Cr and 0.5% C was obtained by mixing ferrochrome, graphite and iron powders. The particle size of iron powder did not exceed 160 microns, and that of ferrochrome powder particles did not exceed 40 microns. A special feature of the production of powdered chromium steel was the use of high-frequency sintering of pressed samples at temperatures of 1100-1300 °C for 10 minutes in an atmosphere of pure hydrogen. After high-frequency sintering, the samples were subjected to dynamic compaction by sediment by 50% at a temperature of 1100 °C in a nitrogen atmosphere. Heating to the precipitation temperature was also carried out by induction, and cooling after precipitation was carried out in air at a rate of 500 C/min. The density of the samples after this treatment was 7.7-7.8 g/cm3. To improve the mechanical characteristics, the samples were subjected to heat treatment, which consisted of quenching from 850 °C in oil, followed by tempering at 620 °C for 1 hour.
Research has shown that induction sintering at 1300 °C ensures uniform distribution of chromium, which leads to an increase in tensile strength to 300-400 MPa. With conventional sintering at 1100 °C for two hours, the tensile strength is 280 MPa at a relative elongation of 3%, which corresponds to the properties achieved with induction sintering at 1200 °C for 3 minutes or at 1300 °C for 1 minute.
Forging sintered billets leads to an increase in tensile strength to 700-800 MPa with a relative elongation of 1-2%. Forging sintered samples gives a tensile strength the same as after forging induction sintered samples at a temperature of 1300 C for 3-10 minutes.
Heat treatment increased elongation to 4-7% with virtually unchanged, and in some cases slightly greater, strength. After heat treatment, the tensile strength of induction sintered samples increased to 880 MPa, while that of conventionally sintered samples increased to 400 MPa.
The authors explain the increase in properties as a result of the use of induction sintering after forging and heat treatment of the samples under study by a more uniform distribution of the alloying element. Almost no undissolved ferrochrome particles are observed in the structure. This does not happen with conventional sintering. This is explained by the fact that during short-term induction sintering, ferrochrome oxides do not have time to form, which during normal long-term sintering form and interfere with diffusion processes.
One of the ways to homogenize the structure of powder chromium steels is to introduce an alloying element in the form of its carbide, for example chromium in the form of Cr3C2, into the mixture of powders. This type of carbide is quite resistant to oxidation and dissolves well in iron when sintered. According to the work, the introduction of Cr3C2 carbide with particle sizes of 5-10 microns ensures the production of a homogeneous structure at a temperature of 1200 °C in 1 hour. According to the work, the homogeneous structure of such materials, providing a tensile strength of at least 700 MPa, is achieved only by sintering at 1280 °C for 1.5 hours. In this case, the maximum strength during single pressing and sintering is achieved with a content of no more than 4% carbide.
The work shows the mechanism and kinetics of dissolution of Cr3C2 carbide in iron and the possibility of using it for alloying powder steels. Studies carried out using X-ray diffraction phase analysis on a material containing 93% Fe and 7% Cr3C2 showed that already at temperatures of 350-400 °C, the interaction of Cr3C2 with iron begins, which consists of the diffusion of iron into chromium carbide. As a result of this interaction, a special carbide (Cr, Fe)3C2 is formed, which begins to dissolve in austenite at a temperature of about 900 °C. The phase composition remains unchanged.
A further increase in temperature leads to the transformation of orthorhombic carbide (Cr, Fe)3C2 into hexagonal carbide (Cr, Fe)7C3. Since the solubility of iron in hexagonal carbide is much higher than in orthorhombic carbide (iron can replace up to 50% Cr in Cr7C3 carbide), the resulting carbide is enriched in iron and, after saturation to values close to the limit, its dissolution in the iron matrix begins. According to dilatometric studies, the process of dissolution of carbides and homogenization of the solid solution is completed at a temperature of 1250 °C in 20 minutes.
In the work, wear-resistant steel was produced using the dynamic hot pressing (DHP) method by introducing 11.0-11.2% (Cr, Fe)7C3 (carbon ferrochrome) and 1.0-1.2% graphite into iron powder PZh2M2. After DHP at 1200 °C (t = 20 min), the steel had RH = 880-980 MPa; KS =98*130 kJ/m2; y = 7.55*7.7 g/cm3; 600-620 HV.
To obtain a stable structure and a high level of strength characteristics, it is preferable to use homogeneous alloy steel powders. In the work, hot-stamped parts made from 40X steel powder, obtained by diffusion saturation from point sources, had R = 870-1200 and 1400-1600 MPa; KS = 570-580 and 380-420 kJ/m, respectively, after stamping and after heat treatment.
An increase in the degree of homogeneity of the structure of chromium steels can be achieved by using various methods of chemical-thermal treatment, introducing chromium-containing salts and ferrochrome powders into the material composition. However, the method of strengthening steel by alloying does not always provide the required properties of the structural material. In the work, an increase in the properties of powdered iron-chromium compositions was achieved by combining alloying with obtaining a fibrous structure. The starting material was a mixture of powders containing 98% PZh2M iron powder, 0.8% pencil graphite and 4% PKh30 high-chromium steel powder. The compressed briquettes were sintered at a temperature of 1250 °C.
The sintered billet, which has a heterogeneous structure consisting of an iron-carbon matrix and incompletely dissolved particles of PKh30 chromium steel, was stamped to obtain a gear. During the plastic shaping of the tooth, a fibrous structure was formed in the surface layer. Fibers from PH30 particles that were not completely dissolved during sintering alternate with fibers of the iron-carbon matrix; the direction of the fibers coincides with the direction of tensile stresses in the tooth root.
After heat treatment, high-strength chromium fibers acquired the structure of alloyed martensite with a microhardness of 850-910 HV, and the iron-carbon matrix acquired the structure of high-carbon martensite with a microhardness of 590-630 HV. The resulting structures provided high values of fatigue strength of the gear tooth material and significant resistance to fatigue crack growth.
The fracture toughness of Fe-9%Cr and Fe-0.6% C-9%Cr compositions was studied in the work. It was found that the factor that most strongly influences fracture toughness is the density of powder materials (Fig. 9). An unusual (compared to compact materials) phenomenon of simultaneous increase in parameter and strength is observed. According to the authors, high values of fracture toughness in sintered porous materials are due to a greater extent to the increase in force required to propagate a crack than to the contribution of plastic deformation occurring at the mouth of the crack and preceding its growth (crack opening). The presence of pores, which are stress concentrators, prevents the development of plastic deformation.
Consequently, in powder materials, when using optimal strengthening by alloying, it is possible to significantly increase not only the strength (yield strength), but also the value of fracture toughness.
Chromium powder steels are widely used as wear-resistant and anti-friction materials. According to the Institute of Applied Mathematics of the Academy of Sciences of Ukraine, the amount of volumetric wear decreases when special chromium carbides are introduced into powder steels. Steels containing carbide, Cr)23C6, had the greatest wear resistance. The addition of chromium in the form of Cr7C3 carbide also helped to increase the wear resistance of the material. Vane pump stators made from this material had higher wear resistance than serial ones made from hardened steel ШХ15.
According to the results of studies conducted at the Institute of Applied Mathematics of the Academy of Sciences of Ukraine, alloying iron-graphite material with chromium increases its wear resistance (Table 9). The MPK-4 material, containing 2% Cr, has the same wear rate as chrome-plated cast iron. MPK-5 material, alloyed with 6% Cr, has higher wear resistance. However, the remaining antifriction properties of powder materials with inclusions of chromium carbides in the structure are insufficient. Such materials cannot be used for the manufacture of piston rings: the material does not break in well, the friction coefficient does not stabilize for a long time, its value increases from 0.065 (with a content of 2% Cr) to 0.107 when the chromium content increases to 6%. With an increased chromium content (6%), the wear of the counterbody material is 1.5 times greater compared to gray cast iron. The increased wear resistance of powder steels, by analogy with cast steels, is associated with their greater resistance to tempering.
The influence of chromium on the tribological properties of the iron-graphite material ZhGr1, obtained by mechanical mixing of components, was studied in the work. The amount of chromium in the ZhGr1 material varied from 2 to 10% (Table 10). The mixing time, depending on the chromium content, was 16-20 hours in alcohol and 4-8 hours dry. The compositions were pressed at a pressure of 780 MPa and sintered at a temperature of 1150 °C in a stream of hydrogen. The porosity of the materials after sintering was 10-12%.
The introduction of chromium into iron-graphite materials (Table 11) slightly increases the hardness of powder materials, since with increasing chromium concentration the volume fraction of primary carbides, the average size of which is 18-22 microns, increases. Under the influence of loads when testing in oil, large carbides easily chip away, creating significant centers of destruction and thereby reducing wear resistance and increasing the coefficient of friction of the material, since it is known that only small, evenly distributed carbides that accept friction loads are capable of increasing antifriction properties. The wear resistance of the material also depends on the phase composition of the carbides. Complex carbides (Fe, Cr)23C6, containing about 70% Cr, apparently have the same low energy consumption as pure chromium carbides Cr23C6. The “bulging” role of chromium when alloying antifriction alloys due to the destruction and chipping of chromium carbides due to their low energy intensity is noted in the work. In addition, the discrete nature of the contact during friction leads to a significant increase in temperatures in individual micro-areas up to several micrometers deep.
In this case, as a result of spot hardening, as shown by X-ray diffraction and metallographic studies of friction surfaces, friction austenite is formed, which creates areas of brittle fracture and adversely affects the antifriction properties.
Additional saturation (3 hours) of chromium-alloyed iron-graphite material with carbon in a solid carburizer at a temperature of 920 °C, followed by quenching and low tempering, increased the hardness and amount of carbides uniformly distributed in the troostite and troostomartensitic structure. As a result, the antifriction properties and wear resistance of heat-treated steels when tested in oil are significantly increased compared to sintered ones (Table 11).
However, compared to unalloyed iron graphite, the antifriction properties and wear resistance of heat-treated chromium steels are at a lower level, which, as in the case of sintered steels, is, firstly, explained by the presence of large carbides (20 microns or more), and secondly, in all alloyed hardened steels, after 50 hours of testing, the v-phase (friction austenite) was recorded in the surface layer in an amount of 30-50%. Friction austenite, having high hardness and brittleness, impairs the bearing capacity of surface layers.
It is known that improvement of the anti-friction properties and wear resistance of high-chromium steels can be achieved by introducing carbon not by mechanical mixing, but by carburizing in a solid carburizer at a temperature of 920 ° C for 5 hours. Thus, the maximum load before setting increases from 3 to 8.8-10 MPa, and the coefficient of friction decreases from 0.4, to 0.1 and from 0.2 to 0.07, respectively, when tested dry and in TC-1 aviation fuel. Since the hardness of carburized steel ZH10 is close to the hardness of the counterbody made of cast steel X12M, setting occurs by point pulls, contact welding, and scratching of the material without traces of gross destruction and smearing.
The wear resistance of the carburized composition ZHKH10 when tested in spindle oil at a load of 10 MPa and in aviation fuel TC-1 at a load of 6-8 MPa for 20 hours is significantly higher compared to the wear resistance of powder steels of a similar chemical composition obtained by mechanical mixing (Table 12) .
The tendency of powder steels obtained from mechanical mixtures of pure components to form a heterogeneous structure can be used as a positive property in the creation of wear-resistant materials with a nonequilibrium coarse heterogeneous structure. This heterogeneous structure eliminates microsetting and increases the wear resistance of materials.
An example of such materials is powdered structural chromium steel grade ZhCh25KhZ, which is more wear-resistant than steel ShKh15 and cemented steel 20Kh. The sintering temperature of these steels is chosen such that homogenization of the material by chromium does not occur. The structure of the material is pearlite ferrite with relatively large ferrochrome inclusions, which have a high hardness compared to the metal matrix. The physical, mechanical and frictional characteristics of ZhCh25KhZ steel after quenching and tempering are given in Table. 13. Created as an analog (in chemical composition) of steel ShKh15, powder steel ZhCh20KhZ has greater wear resistance, despite the fact that it has a porosity of 10-12% and is inferior to it in strength and hardness. Tests in the boundary friction mode with R9 steel at a speed of 2.5 m/s and a pressure of 3.4 MPa showed that the ZhCh20KhZ material is 5 times more wear-resistant than ShKh15 steel. The industrial use of powder parts for the oil pump of forging and pressing equipment, made of ZhCh20KhZ steel, made it possible to increase the service life of the oil pump from 3 to 12 thousand hours.
Chrome vs Stainless Steel: What's the Difference?
Chrome plating Steel and stainless steel products have literally flooded the markets. They are used to make buildings, automobile parts, kitchenware, and many other applications.
What to choose: chrome steel or stainless steel?
This is the question you are likely to ask. Although they may appear to be the same thing, there are many differences between them.
Additionally, some applications may require the use of chrome steel, while other applications may be suitable for stainless steel.
Definition: Chrome vs Stainless Steel
What is chrome steel
Chrome steel is any steel that has been mixed with chromium to prevent rusting. This type of steel undergoes a process known as chrome plating.
It is thanks to chrome plating that a certain percentage of chromium is applied to the surface of the steel metal.
The result is a shiny steel metal that is attractive to the eye. In addition to appearance, chrome plating plays some protective role. Prevents rust and corrosion of steel.
What is stainless steel?
Stainless steel is a type of metal alloy that includes steel mixed with other elements such as chromium, carbon, molybdenum, nickel, silicon and aluminum.
These elements are mixed in a certain percentage, resulting in different grades of stainless steel. For example, one steel contains 10% nickel and another contains 12% nickel.
You should not expect two grades of stainless steel to have the same properties.
The presence of nickel increases the strength of stainless steel. Steel will not scratch or corrode easily. Stainless steel is also rust-resistant.
General concepts about steel grades
We will consider markings that were developed back in the USSR and are now actively used in Russia and in all neighboring countries. It is universal in that it includes all classes, of which there are a lot. Basic moments:
The number is assigned to the entire batch, and a stamp is affixed (paint, by engraving) to each individual product. It consists of numbers and letters, no symbols.
Sometimes the abbreviation “St” is indicated at the very beginning, that is, “steel,” but this is not at all necessary.
Typically, the initial numbers indicate hundredths of carbon, while the letter designating this substance is not placed, since carbon content is one of the fundamental characteristics of alloys. For example, if 20 is indicated, then the content is 0.2%.
Now in more detail with an example:
We have letters (Russian or Latin, as in the sample), they indicate the alloying element that is in the composition. If you need a method for determining the grade of metal without reference books, then you will need to fill in the most common abbreviations:
A more complete list can be found in regulatory documents. By the way, it is interesting that GOST standards for the production of steel alloys, adopted back in the Soviet Union, are still in force today, as are the marking rules. In total, the nomenclature includes more than 1,500 individual values - this is the number of varieties of metals in this category that are manufactured all over the world. It is not surprising that in such a variety it is very difficult to determine by eye what kind of material is in your hands.
We figured out the letters, now the numbers. Everything is simple with them - the first one belongs to carbon, and then we read from left to right: a letter, and behind it a digital indication - what proportion (as a percentage) of the substance is in the composition.
How to distinguish stainless steel from chrome?
Undoubtedly, distinguishing stainless steel from chrome is not an easy task. At first glance, the two metals may seem the same.
The good news is that there are ways to tell the difference between chrome steel and stainless steel.
One way is to use a magnet. If a piece of magnet sticks to the metal, it is not stainless steel. This is because stainless steel is considered non-magnetic. On the other hand, chrome steel can hold a piece of magnet.
Another way to differentiate chrome steel from stainless steel is by appearance or appearance.
Chrome steel has a bright and shiny appearance, while stainless steel is known for its satin appearance.
However, using a visual method to differentiate between chrome and stainless steel can also be misleading.
This is possible because certain types of metal finishes can make stainless steel look bright and shiny. You might assume it's chromed steel, but it's not.
If you are unsure, simply consult a metal specialist for a clear and concise explanation.
How is steel grade determined?
During the production cycle, everything is simple and clear - metal blanks are purchased in rods, bars, sheets or strips. On one of the edges they are marked (engraved with numbers and letters) and have a special color - color is also a signal for metallurgists. In production, the bulk of the metal is used first, and this marked tip is used at the very end. But at home, craftsmen often buy steel secondhand or in such a form that the brand cannot be recognized. Therefore, below we will give several elementary ways to determine the material. To do this you will need:
Durability: Chrome vs Stainless Steel
Is chrome more durable than stainless steel? With proper care, chrome steel can serve you for many years. It is durable when used correctly and in the right conditions.
The ideal environment for chrome steel is dry areas. This location should also be free of elements that can cause crevice and chemical corrosion.
Once chrome steel penetrates, there is a high chance that it will begin to rust. There are many things that can penetrate the surface of chrome steel. These include jigs, door frames, and metal tools.
Once the rust process has begun, it can quickly spread under the chrome layer.
Chrome steel is not completely reliable in terms of durability.
On the other hand, stainless steel has undoubtedly proven itself to be one of the strongest metals. I will serve you for many years without damage from corrosion or rust.
Stainless steel products tend to be expensive, and this is due to the strength of the metal. This feature also makes it ideal not only for a wide range of applications, but also for heavy duty and hazardous applications.
Corrosion and its features.
I noticed that while describing the qualities of stainless steels and noting their need and usefulness for industry, I have not yet focused on why they are so important.
The main property of stainless steels is the ability to resist corrosion, so a few words about what it is. Corrosion is the process of destruction of the surface of metals as a result of purely chemical or electrochemical action of the external environment, usually aggressive. In general, metal corrosion is accompanied by the formation of destruction products on the surface, such as rust, but there are also destructions without external manifestations. The intensity of corrosion depends on the properties of the metal and the degree of aggressiveness of the environment.
Corrosion is a fairly broad concept and is characterized by various manifestations:
- continuous (uniform) corrosion, when the entire surface of the metal is destroyed;
- point (local, crevice, pitting) corrosion, when individual areas of the metal surface are destroyed;
- intergranular corrosion, when corrosion spreads deep into the product along the grain boundaries;
- stress corrosion (corrosion cracking), when cracks develop on the metal surface due to the simultaneous influence of tensile stresses and an aggressive environment.
A separate type is electrochemical corrosion, when electrochemical processes at the interface are added to the purely chemical processes of interaction between the metal and the environment. This is the most destructive type of corrosion.
In the process of electrochemical corrosion, the destruction of metals occurs under the influence of electrolytes and is accompanied by the transition of atoms. In practice, most often electrolytes are aqueous solutions of salts, acids and alkalis. Thus, metal containers, pipelines, machine parts and parts of structures in contact with sea and river water, as well as groundwater, are subject to intense destruction by electrochemical corrosion.
From the theory of electrochemical corrosion it follows that very pure metals have the greatest resistance. But in real life, the use of pure metals is practically impossible, so there is a need to ensure a homogeneous structure of the solid solution in alloys.
Increased resistance to uniform corrosion in a wide range of corrosive environments of varying degrees of aggressiveness is a distinctive feature of stainless steels and alloys. Many types of stainless steels are also resistant to intergranular and pitting corrosion and corrosion cracking.
Is chrome more expensive than stainless steel?
In terms of cost, you will pay less for chrome steel than for stainless steel. This is expected since stainless steel has many more desirable properties than chrome steel.
As we have already said, one of the properties is strength and durability. Stainless steel is stronger and more durable than chrome steel.
So, if you are on a budget, chrome steel should be the metal of choice. However, this will still depend on the application of the metal.
Pros and Cons: Chrome plating of steel versus stainless steel.
Let's look at the advantages and disadvantages of using chrome or stainless steel.
Pros of chrome steel
-Shiny and attractive finish
-No more expensive than stainless steel
— Has elements of modernity
-Durable when used correctly
Disadvantages of chromium steel
-Requires regular maintenance to maintain its shiny appearance
-Can be easily scratched
-Not as durable as stainless steel
-Shows fingerprints and even dust particles
Pros of stainless steel
-Has excellent corrosion resistance
- requires less maintenance than chrome steel
-Available in different classes
Minuses
-Does not look less visually attractive than chrome steel
-It creates visible fingerprints and dust.