The organization of technological operations in production in one form or another involves the preparation of equipment. These are devices that play an auxiliary but important role. Such devices include crucibles. As a rule, these are small cone-shaped containers in which heat treatment operations are carried out. What are crucibles in a technological process? This is equipment, thanks to which various materials are processed in order to give them certain performance qualities.
DIY crucible
To melt metals, special heat-resistant bowls called crucibles are used. They are very popular in jewelry workshops, laboratories and the metallurgical industry. But for a full-fledged process, it is not enough to acquire a simple object with a heat-resistant surface, because different iron requires its own product, which must correspond to the chemical composition and be suitable for a specific temperature regime. These facilities also produce a finished alloy, which remains to be given the correct shape.
Sometimes it happens that such devices may be needed in private business, but purchasing them is expensive. Therefore, it is more profitable to make a crucible with your own hands - you can save a significant share of the budget. Yes, the procedure will require certain skills and patience, but in the end you will get a vessel that is not inferior to factory analogues. It is also important to decide on the types of objects to be melted in order to make a suitable bowl. If you plan to work with different metals, it is recommended to create several products.
Lead crucible
Crucibles of almost all common types can be used in molten lead, with the exception of fragile structures. For example, ceramic, porcelain and clay models are not recommended for use in this case. Of course, the same graphite crucible may be optimal, but its high cost limits the possibilities for application. It should be noted that the crucible for melting lead is not so common in the professional sphere as in private households. Metal is used to make shot, weights, various molds and other devices that may be needed on the farm. The optimal solution for such melting of a house may be a combined composition of graphite with affordable construction additives.
General manufacturing steps
To begin with, the raw materials are prepared, and here everything depends on the model of the future tank. It is better to take components with a reserve, because the first thing is unlikely to work out. Also, for safety reasons, you should carry out production away from open fire, and choose a well-ventilated area.
It is safer to carry out work in a garage or special extension.
The second stage is mixing the materials and giving the casting the necessary parameters. For these purposes, plaster molds are used. Creating the outlines is not difficult, and such information is easy to find on the Internet. Then a homogeneous material is pasted over the outer part of the model, forming a future homemade refractory crucible. It is also important to give it the required depth and thickness.
Clay crucible in the drying process
And the last step is the drying process: the workpiece is placed in a cardboard box and covered with a lid. This will allow the casting to dry and remove excess water from it. Sometimes heat treatment may be required, however, the important point is to control the annealing temperature and protect the skin of the hands and face. If the heat is too intense, the item will burst and there is a chance of severe burns. Detailed instructions on how to make a crucible yourself and at home will be described in the following chapters.
Making a clay crucible
Here you can’t do without fireclay clay, which is sold in any building materials store. It tolerates extreme thermal effects very well, is cheap and there are unlikely to be any problems finding it. As a last resort, you can make a crucible from crushed fireclay bricks. You will also have to buy liquid glass and mix all the ingredients for a homogeneous base. The proportions look something like this:
All components are added in stages: clay and fireclay are mixed until smooth, and water is gradually added to them. The main goal is to create a mixture that will not stick to your hands. When the required consistency is obtained, glass is added and everything is thoroughly mixed. The main thing here is to bring the object to a state where the plane stops cracking. The mixture is ready, and for storage it is recommended to use thick cellophane, or wrap it in 7-10 layers of film.
Before sculpting, you will have to remove any remaining air by hitting the substance about 8-12 times on a hard surface.
The mixed material is applied inside the model, its depth and thickness are formed. It is better to create a semicircular bottom, which will give a greater effect during future melting of iron filings. Also, the substance must be pressed tightly against the model so that air does not form between the planes, and for greater convenience it is recommended to wet your hands with water.
Afterwards, the tank is sent for drying: it is placed in a container made of cardboard or plastic and placed in a dry place. A few hours will be enough to remove any remaining moisture. Also, the product will settle a little, and it will be easy to remove it from the mold. A refractory vessel made of fireclay bricks will be enough for a long time of use, however, the last point of creation should be the firing procedure in a furnace and at T = 800 °C. And the thing can be used for its intended purpose. For ease of use, you will need a crucible furnace, which you can make yourself. For simple installation, you can weld a structure from several pipes to form a cylinder. Usually it is fixed on two parallel posts so that it does not touch the ground. And here the thickness of the walls (minimum 5 mm) and the stability of the product are taken into account (it must easily withstand T = 1600 °C or more).
How to make a graphite crucible
Capacity of this category has many advantages:
If you take the easy route, you can take a graphite rod, and the crucible is almost ready. All that remains is to attach the bottom.
Graphite crucibles in various sizes
If the required tube is not found, everything can be done using two molds of different sizes, which are inserted one into the other, and the free space will allow you to give the desired dimensions. Initially, you need to pour the mortar into an empty container, and you should not spare it. The fact is that the powder will compact and settle. Next, add liquid glass (about 15 ml) and mix everything thoroughly. It is recommended to place the mixed mass in a large cylindrical container (you can use a plastic cup) and press a hole into a small one, leaving the bottom thick enough.
As a result, a vessel will come out, which is given time to dry. In this case, heat treatment will also be required to remove excess liquid. If all the steps were correct, then you will have a high-quality graphite crucible, made by yourself.
Assembling a cast iron crucible
This type is the worst, but sometimes it brings good benefits. All that is required is to place a cast iron glass of a smaller diameter in a metal bowl, and fill the free space with sand and clay.
DIY cast iron crucible
Next, everything is heated in the oven until the mixture melts and takes on the same type of substance. Afterwards, the cup will harden, and iron can be melted in it. This is basic information on how to make a crucible at home and at minimal cost.
Source
Crucible: purpose, principles of melting, manufacturing, options, diagrams
Author: Yuriy Fedorovich Kolesnikov, thermal power engineer*
© When using site materials (quotes, images), the source must be indicated.
Crucible is a vessel for melting metal. As a rule, conversion metal is melted in crucibles, i.e. already brought to the required degree of quality for casting into a mold or refining (deep purification from impurities). The general line of development of large-scale metallurgy is to reduce the number of processing steps, up to the release of conditioned metal directly from the melting furnace, but in industry crucible melting still retains significant importance, and in handicrafts and jewelry it dominates.
A little about melting
In a deep vacuum, the high-purity metal being melted can be heated exactly to the melting temperature or slightly higher, and kept at it for some time so that tiny, literally a few atoms, remains of crystallites melt. Then the metal can be allowed to cool slightly below its melting point - it will remain liquid, like a supersaturated solution without a seed crystal. If we now pour the metal, also in a vacuum, into a mold made of a chemically absolutely inert material, in which a seed crystal of the same metal is placed, then, observing all the subtleties of this technology, we will obtain a single-crystal casting with unique properties.
In amateur conditions, vacuum melting, alas, is not feasible. In order to properly make a crucible for melting metal yourself, you need to take into account a number of features of melting in a non-inert chemical gas environment. The melted metal, firstly, interacts with air, causing part of it to be lost to the formation of oxide, which is especially important when melting scrap precious metals: at its melting temperature (1060 degrees Celsius), even gold noticeably oxidizes. To compensate to some extent for oxidation, the crucible must provide a reducing environment for the melt or be chemically inert if the metal is melted with a clean open flame, see below.
Secondly, so that the metal in the crucible does not freeze until it is brought to the casting mold, so that the remnants of the original crystallites do not spoil the casting, and the melt acquires sufficient fluidity, the metal in the crucible is overheated. For example, the melting point of zinc is 440 degrees, and its foundry temperature is 600. Aluminum, respectively, 660 and 800. Since overheating of the metal after melting takes some time, degassing of the melt also occurs at the same time, this is the third thing.
Recovery
In metallurgy, atomic carbon C, carbon monoxide CO (carbon monoxide) and hydrogen H are used as reducing agents. The latter is most often an accidental guest, because for this purpose it is too active and is absorbed by metals without forming chemical compounds with them in large quantities, which spoils the casting material. For example, solid platinum at room temperature can absorb up to 800 volumes of hydrogen. A platinum blank in a hydrogen atmosphere literally swells before our eyes, cracks and falls into pieces. If you take them out of the hydrogen chamber and heat them, hydrogen will be released back.
Note: in a similar way, but in smaller quantities, metals absorb/emit other gases, e.g. nitrogen. This is why degassing of the melt is required, see also below.
A noticeable proportion of hydrogen reduction occurs when heated by an open flame of a gas burner, upon its contact with a less heated surface. The metal does not deteriorate - the absorbed hydrogen is released and burned later in the smelting process. But, if the crucible material is also prone to gas absorption, it may crack and burst during melting; this must be kept in mind.
CO reduction is noticeable if the metal in the crucible is melted by the open flame of a liquid (gasoline, kerosene, diesel) burner, for the same reasons. Liquid fuel burns much slower than gas, and its afterburning zone extends several cm from the burner nozzle. Reduction with carbon monoxide is the cleanest from the point of view of the metal: it does not spoil the metal and does not produce by-products with a strong excess of the reducing agent. Therefore, CO reduction is widely used in metallurgy when smelting metal from ore, but no one has yet figured out how to make a crucible furnace (see below), in which oxidation compensation would be completely provided by CO.
Atomic carbon is a reducing agent energetic enough to compensate for oxidation. It is also not difficult to create a reducing environment in a crucible using C: it is enough to introduce free carbon in one or another allotropic modification into the composition of its material or make the entire crucible from a heat-resistant and mechanically sufficiently strong allotrope C; graphite is one of them. When reducing C, there is a danger of carburization of the melt, but graphite releases very little atomic carbon when heated. If you heat the metal in a graphite crucible with a gas flame, then the excess C will immediately find a more “tasty” H for it and the danger of carburization will be reduced to zero. And for other heating methods (see below), you can select the dimensions, configuration of the crucible and the addition of graphite to its material so that there is simply no excess C under any conceivable melting mode. This is a very valuable property of graphite, keep it in mind too.
Note: the coefficient of thermal expansion of TKR graphite is negative, which significantly compensates for the thermal expansion of the crucible, increases its durability and increases its service life. Also valuable quality.
Excerpt
So, it’s clear why the melt in the crucible needs to be overheated and held. Although metal casting is a completely different topic, it still needs to be mentioned here that the melt holding time should be observed quite accurately. Chemically pure metals are almost never used in practice, for example. gold 9999 wears out very quickly; The exception is electrical copper and zinc for galvanizing, the cleaner they are, the better. Most often they use the so-called. eutectic alloys; eg steel is a eutectic of iron and carbon, and duralumin is a complex eutectic of several components. If the melt is allowed to sit, the structure of the eutectic in the casting will change and the finished product will be spoiled. The holding time is especially critical for bronze and brass: they need to be cast immediately, as soon as the play of the melt in the crucible apparently changes and becomes calmer. Remember how the engineer Telegin in A. N. Tolstoy’s “Walking Through Torment” was worried that the bronze would not wear out?
In relation to the manufacture of a homemade crucible, degassing of the melt during exposure is significant in that at this time it (the crucible) experiences significant dynamic loads from bubbles of released gases and/or the play of the melt itself. That is, make the crucible withstand a large amount of thermal deformation and, if recovery is required, a small amount. Its material must also be viscous enough to withstand shock waves from bursting bubbles and shocks from melt jets. It is this circumstance that explains the low durability and reliability of homemade graphite crucibles (see below).
Making a crucible for melting lead with your own hands
To give the prepared mass the required shape, you can choose one of the options presented below. They look like this:
- use a collapsible or destructible plaster mold;
- blind yourself.
Which option to choose is up to you. The last stage of creating a crucible must be performed strictly according to the specified algorithm:
- After the vessel has been formed, it must be dried.
- As soon as it dries, place the workpiece in a muffle furnace. Set the temperature to 800 degrees and bake for one or two hours. This temperature is necessary so that the glue can melt and firmly bind all other components together. If the temperature is lower, the crucible will fall apart during the first operation in the furnace due to the temperature of the contents. If the temperature is higher, it will fall apart before the end of firing.
The resulting form can withstand heat of 1600 degrees. If the materials have been properly processed and milled, it will last up to 30 meltings.
Go to page
German Kuzya
White and fluffy
Kaolin-charcoal crucibles.
Kaolin (pure enriched kaolin). 10 Pots that were smelted, thoroughly cleaned of slag (or fireclay). 5 Hardwood charcoal* (powder). 5 If the crucibles are well packed, they keep the temperature at 1550-1650°C. * Later P.P. Anosov replaced it with graphite.
Kaolin-carborundum crucibles.
Kaolin (pure enriched kaolin). 1 Carborundum (this is silicon carbide). 1 Graphite (powder). 1
Then press it. If the crucibles are well packed, they maintain a temperature of 1800-2000 without problems. During pressing, coat the punch with graphite powder and use as little water as possible when preparing the mass. If, during pressing, water flows from the mold or at least stands out at the bottom, more than two or three drops, this is bad, there is a lot of water in the mass and the crucible may not achieve optimal resistance later.
Is it possible to replace graphite with coke in crucible mixtures?
It is advisable to abstain from coke; coke contains a low-melting slag that can destroy the crucible at high temperatures. If you use it, look for the lowest ash, but it is still better to use graphite or charcoal. And if coke is the only alternative, then take pieces of large coke that are very reluctant to crack under the blow of a handbrake (hammer), the harder the coke, the higher the quality and the lower the ash content. And coke, at least a little, contains sulfur, which is also undesirable. If you use charcoal, then also select pieces of dense and hard charcoal and grind it. And lastly, kaolin clay is often called fireclay or porcelain clay. These are different things. In addition to kaolin, china clay contains finely ground quartz and feldspar, and fireclay clay contains a large amount of silica. In both the first and second cases, such crucibles cannot withstand temperatures above 1400°C. Be sure to look for kaolin, and preferably pure kaolin; it is already the weakest link in the crucible mass.
To prevent crucibles from bursting during melting
, the pressed crucibles need to be dried well; I dry mine in a dry room for at least a month. You can, of course, after a week, put it in the oven and dry it for several hours at 80°C, then raise the temperature to the maximum and if everything works out, then leave it for a couple of hours and immediately go into the oven for firing in a muffle furnace at 900-1000 for an hour or two. Crucibles fired in a muffle furnace can be stored in a dry place and used as needed, but before use, they can be heated again in the oven.
Fillers.
Briefly the essence is this. Charcoal powder is porous and when the crucible mass begins to expand when heated, the coal particles are easily compressed, thereby saving the crucible from cracking. Hair or tow works in approximately the same way in a crucible mass. Graphite powder, as the best material, is well suited for these purposes, but it has a drawback: graphite does not shrink when heated and the crucible mass requires a filler so that it does not crack when heated. It turns out that in addition to graphite, in addition to the binder, you also have to add a neutral filler (fireclay chips, corundum or carborundum) to the crucible mass, which sometimes negatively affects the durability of the crucible. As a filler to protect against cracking, you can use either short-cropped hair or tow no longer than 5mm-10, otherwise the hair or tow will clump into clumps. The charcoal has already gone through the pyrolysis process and will not gas, because there is nothing to use. If you use charcoal instead of graphite, then there is no need for fireclay chips, but the charcoal itself is quite porous. In this case, you have to increase the amount of binder, which is also not good. It turns out that you need to mix it with the same fireclay, but its presence greatly reduces the durability of the crucible.
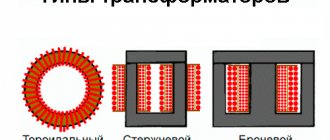
What to make from
Melting crucibles are made (see figure below):
Crucibles for melting metal from various materials
Their comparative characteristics are as follows:
Note: graphite, cast iron and steel crucibles for use in induction furnaces (see below) are completely unsuitable, because completely absorb EMF energy.
About graphite crucibles
Graphite crucibles are made either turned from massive natural graphite (expensive), or sintered at high temperatures from graphite powder (cheaper, but still not very cheap). Hobbyists often try to make “graphite” crucibles from ground graphite with a kaolin binder, etc., but what they end up with is not graphite, but overly graphitized ceramic crucibles - fragile, withstanding no more than 10 melts and spoiling the metal due to excessive release of atomic carbon by finely dispersed graphite . A more or less rational way to use ground graphite in amateur crucible melting is to make a tabletop mini crucible furnace from it for ceramic neutral crucibles, see fig.
Heating methods
If you need to melt more than 150-200 g of metal at a time, then you will need to build a crucible furnace next to the crucible, otherwise it will be very difficult to achieve homogeneity of the melt and high quality casting. The exception is low-melting and easily recoverable lead: up to 20-30 kg of it can be melted at a time at home. A relative exception is zinc for hot galvanizing; its melt in a crucible without a furnace can be up to 2-2.5 kg, but borax must be sprinkled on top of it so that the surface of the melt is completely covered with its fluidized layer. Steel fasteners are thrown into the melt through a layer of borax.
The optimal method in all respects for heating the crucible in a furnace is with gas, pos. 1 in Fig., but a gas crucible furnace is a rather complex structure, although it can easily be made independently. The most suitable crucible for a gas furnace is a graphite ceramic crucible, because its material has fairly high thermal conductivity. If there are particularly high requirements for metal purity, it is better to use a neutral ceramic crucible. When lower for fusible metals - cast iron, as it conducts heat better and thereby saves fuel. Graphite crucibles are placed in a gas furnace only if strong reduction of old oxidized metal is required, and the danger of carburization is insignificant, for example, when melting silver extracted from the earth for refining
Methods for melting metal in a crucible
For low-melting metals, the electric crucible furnace, pos. 2; it may be the so-called ohmic (with heating by a nichrome spiral) or induction, with heating from an electromagnetic oscillation generator, see below. Only ceramic neutral or, to a limited extent, graphite crucibles are suitable for induction furnaces.
If the crucible contains more than 2-2.5 kg of metal, then according to safety rules the crucible furnace must be made tiltable (item 3), because and 1 kg of melt spilled on the floor is already a big disaster. On the contrary, it is preferable to heat metal in small jewelry crucibles without a furnace, directly with the flame of a burner, pos. 4. In this case, the crucible is held throughout the melting process with a special spring grip, pos. 5 and 6.
Note: silver and its alloys, as well as lead on sinkers, can be melted at home in quantities of up to 15-20 g, using instead of a crucible... a food-grade stainless steel spoon, see fig. on right. For safety, then it is necessary to make gaskets for the jaws of the vice with longitudinal cuts under the handle of the spoon. The flame is exclusively gas; gasoline can burn a spoon.
Electric heating
Ohmic crucible furnaces are mainly used for smelting lead or tin. For more refractory metals, they turn out to be uneconomical, but up to 20 kg of lead can be melted at a time in a home crucible electric furnace; how to make your own electric crucible for melting lead, see for example. video:
Video: electric crucible for melting lead
Melting aluminum in a crucible turns out to be more profitable by induction due to its high electrical conductivity, but this trick no longer works with copper - its temperature and latent heat of fusion are much higher. With the induction melting method, the metal is heated by Foucault eddy currents, for which the crucible with it is placed in an EMF coil of thick copper wire, powered by alternating current from an electromagnetic oscillation generator. How to make a generator with your own hands for inductively heating small amounts of metal, for example, for trinkets, is described in other materials, or, for example, see next. video guide.
Video: DIY induction heating
Induction crucible furnace for aluminum melting
With an increase in the amount of melted metal, not only does the required power of the generator increase, but its optimal frequency also decreases, this affects the so-called. surface effect (skin effect) in metal. If 100-200 g of aluminum can be melted into EMF from any homemade generator for inductive heating, then installation of 1.5-2 kg of duralumin or magnesium alloy is already a solid structure, see fig. on right. If you intend to work with aluminum, then think carefully - is it worth building something like this? Wouldn't it be easier to use a mini gas furnace for melting small quantities of aluminum alloys, see for example. video clip
Video: mini furnace for melting aluminum
Aluminum melting crucible
Aluminum can be processed using graphite crucibles, which are capable of providing melting temperatures in the range from 400 to 1400 °C. In particular, so-called mammut crucibles are widely used. The widest temperature range for aluminum melting is offered by universal containers. They provide both intense heating and direct melting. In the middle temperature range, an aluminum crucible is used, immersed in an electric furnace. Such forms, in addition to temperature resistance, are distinguished by protective coatings against corrosion and mechanical durability. The most stable crucibles for servicing aluminum are used in gas furnaces, which provide a temperature regime of 1400 °C. Less commonly practiced is the use of a combination of crucibles with liquid fuel furnaces.
Making crucibles
Now it's time to make your own melting crucible. From the above it is clear that it makes sense to make crucibles with your own hands:
There is nothing special to say about steel crucibles - they are just a steel vessel with a welded handle. Steel crucibles are used for melting low-melting metals; sometimes - zinc for hot galvanizing with quality up to 3+. Steel crucibles for lead, tin and zinc are only suitable for melting one specific metal, because... after 1-2 melts they themselves are covered with it from the inside.
Ceramic neutral
The composition of the mixture for forming a neutral ceramic crucible is 7 parts of fireclay clay, 1 part of finely ground fireclay (up to the fraction
Source
Clay crucibles
The popularity of clay crucibles is due not so much to their positive performance qualities as to their availability and ease of manufacture. Typically, for such needs, fireclay clay is used, which during the manufacturing process is subjected to three stages of processing - creating a working mass, modeling and drying. This technology has replaced porcelain containers. They could also be molded without much effort, but in practice they did not meet the requirements for strength and resistance to high temperatures. As a result, cracks and chips appeared. What are clay crucibles in practice? This is a stronger material than porcelain, even at elevated temperatures. However, in terms of other qualities of the same interaction with metal alloys, clay is not so harmless - at least compared to alundum and graphite products. Nevertheless, economically, the use of clay crucibles more than justifies itself when it comes to small typical melting operations.
How to make a crucible or melting furnace with your own hands
Almost every item has several types and purposes, including stoves. There are stoves for heating rooms and for cooking food, and there are special devices for melting metals or storing them in molten form. Such devices are called crucible melting furnaces. They have a specific purpose and therefore the list of enterprises where they have found their application is quite small. These are mainly factories and laboratories. But what to do if you need to melt metal for some purpose at home? Buying such equipment is very expensive, but it is quite possible to make it yourself. This requires minimal knowledge in this area, desire and time.
Making a crucible with your own hands
Making a crucible is not a labor-intensive task. To make it yourself using martel you will need:
- crushed graphite;
- whole graphite;
- graphite tube;
- fireclay martel;
- magnesite;
- felt.
Technological sequence:
- Take thick paper. Roll it into two cylinders of different diameters. The outer cylinder is hollow and larger in diameter, while the inner cylinder is closed on both sides and smaller in size.
- Mix the martel and other ingredients in a separate container. Next, mix with liquid glass until a homogeneous mass is obtained, the consistency of which is comparable to shortbread dough.
- Part of the resulting mass is distributed on a flat and smooth surface. Then place paper cylinders on it one inside the other to obtain the shape of a crucible. The distance between the paper cylinders is the thickness of the crucible walls.
- The remaining mass is filled into the prepared form.
- After formation, the paper elements of the mold are removed and the workpiece must be dried a little at room temperature.
- The crucible is then placed in an induction furnace to remove any remaining moisture from the mixture. It should be heated at low temperatures so that the mold does not burst. The process takes considerable time.
- After drying, the crucible is fired at a temperature of no more than 600 °C.
- The quality of the crucible is checked by tapping it, like a crystal glass.
Types of crucibles
A crucible furnace is a container made of refractory material in which metal is heated to a certain temperature.
The main materials from which crucibles are made: Crucible furnaces are used both in factories where metal products are made, and in small enterprises, for example, for the manufacture of jewelry.
Ceramic stoves are the best option. When metals are melted in a ceramic crucible, no changes occur in the substance itself. even base metals or alloys of cobalt, chromium or palladium in such crucibles without any problems
Graphite crucibles. Such furnaces are characterized by a long service life and high resistance to oxidation, which makes them universal for melting any metals and especially alloys based on zinc and brass. In addition, they are often used in induction furnaces. Graphite crucibles can withstand very high temperatures, such as eight hundred degrees to melt aluminum.
Features of Graphite Crucible
Graphite crucibles for melting non-ferrous metals have a fairly long service life. They resist oxidation, thermal and mechanical effects of the melt well. Crucibles are mainly used in conjunction with induction heating furnaces.
Graphite Crucible Induction Furnace
Graphite, as a material, has the following properties:
- heat resistance;
- fire resistance;
- high thermal conductivity;
- increasing strength characteristics when heated;
- low specific gravity;
- low porosity;
- prevention of oxidation;
- resistance to: corrosion;
- sticking;
- burnt.
Graphite crucibles are not made from pure graphite. To form the mixture, refractory clay (fireclay, alumina) and quartz sand are added to the graphite. Some of the clay can be replaced with kaolin. High-quality containers can easily carry a significant number of heats.
For steel melting, the composition of the charge mixture is selected according to the requirements for the purity and characteristics of the steel.
| Batch materials, % | Graphite | Alumina | Kaolin | Chamotte |
| Alloy type | ||||
| High strength steel | 54 | 35 | — | 10 |
| Medium hard steel | 40 | 38 | — | 22 |
| Razor steel | 12 | 40 | 40 | 8 |
| Quality steel (clean) | 3 | 87 | 10 | — |
| Copper | 8 | 67 | — | 25 |
| Brass, bronze | 12 | 50 | 13 | 25 |
| Gray cast iron | 53 | 43 | — | 4 |
| Modified cast iron | 50 | 40 | — | 10 |
For smelting copper and cast iron, Blaninger proposed the following proportions of crucible charges.
| Batch materials, % | Copper alloys | Cast iron | ||||
| A | B | C | D | E | F | |
| Graphite | 48 | 57,5 | 55 | 50 | 58 | 55 |
| Clay | 32 | 25,5 | 35 | 40 | 35 | 30 |
| Kaolin | 6 | 10,5 | 5 | — | — | 7 |
| Quartz sand | — | — | — | 5 | 7 | 8 |
| Chamotte | 14 | — | 5 | 5 | — | — |
| Silicon sand | — | 6,5 | — | — | — | — |
An important production indicator is the cost of finished products with given characteristics. Therefore, various types of graphite are used to make crucibles.
Grain graphite is not used. Lamellar or crushed into a small fraction is used. Large-lamellar graphite is fire-resistant, heat-resistant and has a high density. Fine-plate graphite (amorphous) is less resistant and the number of melts in it is much less. The strength characteristics of crucibles depend on the ash content of graphite.
Graphite crucibles for steels
Crucibles for steels are made of highly concentrated graphite, the content of which reaches 90%. But the presence of iron oxides should be kept to a minimum. When smelting copper, graphite is partially replaced by retort graphite or coke.
Recently, the following grades of graphite have found widespread use:
- EG2 – electrographite;
- GM – fine-grained graphite;
- MPG – isostatic graphite.
Electrographite
Electrographite EG2 differs from the graphite from which electrodes are made in that it has less porosity. This grade is recommended for re-melting or for melting with slag removal.
Fine-grained graphite
Fine-grained graphite GM is recommended for melting pure metals. Resistant to burning.
Isostatic graphite
Isostatic graphites PGMs have the best characteristics, and therefore high cost.
Inductor assembly
The heating element of the crucible arm at home is usually an inductor. It has a cylindrical shape with a cavity inside. A homemade crucible with metal shavings is placed in this cavity. The inductor is made of fire-resistant material , inside it there is a wire winding, most often copper wire is used.
Using a special generator, current is supplied to this winding, which creates an electromagnetic field. Which, in turn, creates an eddy current in the crucible and in the metal placed in it. They melt the chips. The inductor itself is assembled from 4 vacuum tubes with a parallel connection. Such an inductor can be connected to a regular outlet. There is another option for assembling an inductor with your own hands from an electromagnetic core and two layers of winding. The first layer is 10 turns of copper wire with a thickness of 4 mm, and the second is one turn, the material for which is a metal plate with a cross-section of 15 * 5 millimeters. The electromagnetic core is U-shaped and consists of a set of steel plates. The first winding is made around the plates, which is placed in an insulated housing; the secondary winding connects the core and metal bars , between which there should be a distance equal to the dimensions of the crucible. This entire structure is placed in the furnace body.
So, we get a furnace in which the inductor is located. Wires go from the inductor to the socket. A crucible is placed in this furnace in such a way as to enclose the bars. If it is placed correctly, a buzzing sound will be heard, indicating that tension has appeared and melting has begun. If there is no sound, then use the handle to move the crucible until the circuit is completely closed.
Making a crucible from clay
You can make a crucible from fireclay clay. This is an inexpensive option and also highly resistant to high temperatures. This clay is used for laying stoves and can be purchased at any hardware store.
Fireclay clay can withstand temperatures up to 1600 degrees Celsius. So, you will need fireclay clay (sold in bags in hardware stores), liquid glass (sold there) and ground fireclay. It can be bought or made from fireclay bricks.
In order to make a mixture from which a crucible will be fashioned in the future, take 7 parts of clay, 3 parts of fireclay and 10 tablespoons of liquid glass per liter of dry mixture. Fireclay and clay are mixed until smooth. After this, water is slowly added. In order not to spoil the workpiece, you can pour out part of the mixture , and in case of a large amount of water, add dry powder. You need to knead until the clay stops sticking to your hands.
Only after the clay of the desired consistency has been mixed can glass be added. When adding glass, you need to thoroughly knead everything until the clay stops cracking. It is best to add glass to a lump of clay and roll it into a roll, then fold it several times and repeat the procedure until it stops cracking. The material for the crucible is ready. Until the moment when it is used, it must be stored in several layers of cellophane.
There is clay, now to make a crucible you need to take a mold, the easiest way is to use a plaster mold. How to make such a form can be found on any website on plaster modeling. So, directly making the crucible.
Before you start sculpting, you need to knock all the air out of the clay; to do this, you can lay a newspaper on the floor and forcefully throw a lump on it several times, ten times will be enough. Now take a lump of clay and carefully press it into the bottom of the mold, after which the walls of the product are formed in small lumps. Their thickness can be controlled along the edge of the mold. very important to carefully press the clay into the mold so that no air cushions form there. After the crucible is sculpted, you need to make the inner surface smooth. To do this, just moisten the clay with water.
After this comes the moment of drying. The mold with clay is placed in a cardboard box and covered with a lid. After seven hours, all the water from the clay will evaporate and the shape of the future crucible will “shrink” a little, so getting it out of the mold is not particularly difficult. After this, the crucible continues to dry in the same box; as it dries, all defects will be eliminated by themselves and the pot will acquire a gray color. Sometimes small cracks may appear. They can be covered with wet clay. Next, the pots are fired at a temperature of 800 degrees in a muffle furnace. After firing, the crucible is ready for use.
How to make a mixture for a neutral type ceramic crucible?
First you need to prepare the form of a homemade crucible for melting lead. This is done according to the following algorithm:
- Dry clay is mixed with ground chamotte until a homogeneous consistency is obtained. It is recommended to make 20 revolutions in the fireclay mill.
- As soon as the required state is achieved, you can unload the mass and begin mixing by hand, adding water little by little. The mixture, in the right condition, will form a lump and will not stick to the skin or leak through your fingers.
- Now you need to add silicate stationery glue.
- Mix the whole mass thoroughly. It is worth noting that this is the most difficult and time-consuming stage. Continue processing until a homogeneous consistency is obtained.
- The next step in creating a crucible for melting lead with your own hands will be to remove air from the finished mixture. It is necessary to ensure that not a single bubble remains in it. Otherwise, the crucible will burst when overheated. To avoid this, the air must be knocked out.
- Spread a film on the hard floor (never use newspapers!).
- Now the fun begins. To eliminate all the air from the workpiece, it is necessary to throw it onto the film with maximum force. Do this until the bubbles stop coming out of the mass. After that, throw 10 more times (more is possible).
- After the procedure for removing air from the mixture has been completed, it can be stored. To keep it for as long as possible, you should use glass containers with a hermetically sealed lid.
Attention! Do not store the workpiece in a plastic container or wrapped in several layers of film. It will dry out in a couple of weeks and will be unusable!