Eutectic cast iron
“Eutectic is (from the Greek eutektos - easily melting) liquid, which cannot be eliminated in equilibrium with two or more solid phases.
The crystallization temperature of the eutectic is called the eutectic point. The product of crystallization of liquid eutectic is solid eutectic, a highly dispersed mixture of several solid phases of the same composition as the liquid eutectic. “- quote from the reference dictionary. Casting from eutectic (tight) cast iron is durable, homogeneous, has no shells, is resistant to all types of corrosion, can withstand low temperatures of the water being poured, and ensures easy cleaning and maintenance of the boiler. The use of cast iron boilers with high heat capacity makes it possible to use a three-pass design of channels for the passage of heated gases with an optimal configuration of heat transfer fins, reduce the heating temperature of the boiler heat exchanger, reduce the condensation of water vapor from combustion products and achieve complete washing of the surface of the hot chamber with water. All this allows us to greatly increase the efficiency and reliability of water heating equipment.
De Dietrich is one of the largest and oldest European manufacturers of heating equipment. More than 150 years of experimentation in cast iron smelting and more than 100 years of experimentation in the production of cast iron boilers allow the company's more than 3,000 employees active in the heating sector to continue to develop the company's traditions, which invariably lead to success. Production is located in industrial centers mainly in the north-east of France and Germany.
carried out by a connected laboratory. Its industrial base and strict quality control throughout entire production periods satisfy all the requirements of product consumers and GOST. All products undergo strict heat treatment. Metal cutting Pipe making Mohs hardness Weather and folk signs Works of the children of our employees Periodic table of Dmitry Ivanovich Write us a letter Moscow “Monolith” ® ™ tel. fax + ; federal mob. tel. + E-mail: [email protected]
Surface hardening with heating using high-frequency currents is used to increase the surface hardness and wear resistance of cast iron castings. It is recommended to subject pearlitic cast iron to surface hardening. This is explained by the fact that when heating pearlitic cast iron there is no need to saturate austenite with carbon due to the dissolution of graphite. The transformations that occur during surface hardening of such cast irons are similar to the transformations during surface hardening of pearlitic cast irons 840 - 950 o C, heating time - Several moments, heating rate about 400 o C / s, cooling in water or emulsion. The hardness after hardening of gray cast iron is HRC 50 - 55, high-strength HRC 58 - 60. The hardness distribution across the cross-section of the hardened layer (1.5 - 4 mm thick) is fairly uniform. The microstructure of the surface layer is finely needle-like martensite and graphite inclusions. After surface hardening, a short tempering is carried out. Surface high-frequency hardening is applied to parts made of pearlitic cast iron that are subject to wear - machine tool bed guides (made from modified gray cast iron), articulated and cam shafts (made from high-strength cast iron), cylinder liners (made from alloy cast iron) and other parts.
The theory of heat treatment is necessary for every heat treatment specialist to know, since the quality of manufactured parts depends on the correct choice, development of the most effective technological process of heat treatment and its implementation. Only by studying the theory and practice of heat treatment of metals can a heat treatment specialist successfully operate in modern machine-building factories, successfully introduce the latest achievements of science and technology into heat treatment technology, and fight for mechanization and automation of technological processes.
Source
Scope of use of cast iron
Cast iron is used in various industries. For example, it is widely used in mechanical engineering for the production of various parts.
Most often this material is used in the production of engine blocks and crankshafts. To manufacture the latter, an improved alloy with the addition of special graphite impurities is required. This metal is resistant to friction, so high quality brake pads are made from it.
In harsh climatic conditions, cast iron alloy is indispensable, as it allows machine parts made from it to operate smoothly even at the lowest temperatures.
It has also proven itself excellent in the metallurgical industry. Its excellent casting properties and relatively low price are highly valued. Products made from it are characterized by very high strength and wear resistance.
A great variety of plumbing products are made from cast iron alloy. These are radiators, sinks, various sinks and pipes. Cast iron bathtubs and heating radiators are widely popular. Their service life is very long. Many apartments still use these products to this day, because they retain their original appearance for a long time and rarely need restoration.
It is also important that the excellent casting properties of cast iron make it possible to make entire works of art from it: such as openwork forged gates and all kinds of architectural monuments.
It is noteworthy that the price for 1 kilogram of cast iron is determined by the amount of carbon in its composition, as well as the presence of various impurities and alloying components. The price of a ton of cast iron is about 8,000 rubles.
Today there is not a single area where this metal is used. Its castings and alloys form the basis of many components, mechanisms and parts. Sometimes it is used as an independent product, perfectly coping with the functions assigned to it. This iron-containing compound is unique in its kind. It remains indispensable to this day.
White cast iron
White cast iron is a type of cast iron that contains carbon compounds. In this alloy they are called cementites. This metal got its name due to its characteristic white color and shine, which is clearly visible at the fracture. This shine is manifested due to the fact that such cast iron does not contain large inclusions of graphite. In percentage terms, it is no more than 0.3%. Therefore, it can only be detected by spectral or chemical analysis.
Differences between cast iron and steel
Cast iron and steel are compounds of iron and carbon. Their fundamental difference is the percentage of carbon. This chemical element is included in cast iron in the form of cementite or graphite, its content ranges from 2.14 to 4.5%. If the alloy contains less carbon than the stated norm, then steel is formed.
To understand the difference between steel and cast iron, you need to consider their characteristics. A distinctive feature of cast iron is the amount of carbon. Its minimum content is 2.14%. This is the main indicator by which this material can be distinguished from steel.
The iron content in steel is 45%, and the percentage of carbon is up to 2. To determine the differences by eye, you need to pay attention to the color. Steel is light in color and cast iron is dark.
Only chemical analysis can determine the percentage of impurities. If we compare the melting point of cast iron and steel, then for cast iron it is lower and amounts to 1150-1250 degrees. For steel - around 1500.
To distinguish the material, you need to do the following:
- The product is lowered into water and the volume of displaced water is determined. Cast iron has a lower density. It is 7.2 g/cm3. For steel - 7.7−7.9 g/cm3.
- A magnet is applied to the surface, which attracts the steel better.
- The chips are rubbed using a grinder or file. Then it is collected in paper and wiped on it. Steel will not leave marks.
Sources:
- https://tokar.guru/hochu-vse-znat/chto-takoe-chugun-sostav-i-soderzhanie-ugleroda-v-splave.html
- https://stroy-podskazka.ru/chugun/skolko-ugleroda
- https://obrabotkametalla.info/stal/sostava-splava-chuguna-i-otlichie
- https://metatorg.ru/marki-stali-i-splavy/chugun
Composition and types of white cast iron
White cast iron consists of the so-called cementite eutectic. In this regard, it is divided into three categories:
In addition to the above classification, it is divided into ordinary, bleached and alloyed.
The internal structure of white cast iron is an alloy of two elements: iron and carbon. Despite high-temperature production, it retains a fine-grained structure. Therefore, if you break a part made of such metal, a characteristic white color will be observed. In addition, in the structure of a hypoeutectic alloy, for example, hard grades, in addition to pearlite and secondary cementite, cementite is always present. Its percentage can approach 100%. This is typical for a eutectic metal. For the third type, the structure is a composition of eutectic (Ep) and primary cementite.
One of the varieties of such alloys is the so-called bleached cast iron. Its basis, that is, the core, is gray or high-strength cast iron. The surface layer contains a high percentage of elements such as ledeburite and perlite. The whitening effect up to 30 mm deep is achieved using the rapid cooling method. As a result, the surface layer is white, and then the casting consists of an ordinary gray alloy.
White cast iron structure
Depending on the percentage of alloyed additives, the following types of metal are distinguished:
Quite common elements are used as alloying additives. The alloyed white cast iron obtained in this way acquires new, predetermined properties.
The influence of carbon on the properties of cast iron
The presence of carbon affects the properties of the alloy. Brittle cast irons may have increased hardness compared to steel. Unlike steel, they are difficult to weld or forge, and for some varieties these technological processes are generally not applicable. The iron included in the iron-carbon alloy determines its main properties. First of all, the melting point is above 1530°C. This element also gives the alloy magnetic properties. In its pure form, the metal is quite strong and ductile. But its chemical activity towards oxygen prevents its use in technology (the metal quickly rusts when it enters into an oxidation reaction).
The inclusion of carbon and other impurities in alloys significantly increases the durability of products. Part of the carbon enters into a chemical reaction with iron even at the melting stage, forming a stable compound - cementite (Fe3C). Cementite inclusions reduce ductility while increasing the strength and hardness of the resulting alloy.
The strength of steel with a carbon content of about 1% is especially high. When there are more compounds in the alloy, this characteristic gradually decreases, but at the same time the impact strength and the so-called cold brittleness (reaction to temperature changes) decrease.
Carbon can also be contained in a chemically free state (graphite). The presence of graphite inclusions increases fragility, but at the same time creates granularity that prevents mechanical destruction of the surface. Graphite can act as a lubricant, reducing frictional resistance. Graphite inclusions also reduce the effects of vibration. Carbon in its pure form and in the form of compounds can reduce the melting point (in some cast irons by almost 400°C).
Thus, alloys of iron and carbon (steel and cast iron), due to different ratios of elements and their chemical forms, can differ significantly in their properties.
Properties of white cast iron
Any cast iron alloy, on the one hand, is very strong, but at the same time it is quite brittle. Therefore, the main positive properties of white cast iron include:
White cast irons, with a reduced percentage of carbon, are more resistant to high temperatures. This property is used to reduce the number of cracks in castings.
Appearance of white cast iron
The disadvantages include:
Another disadvantage is poor weldability. Problems in welding parts made of this material are caused by the fact that during welding, cracks form, both during heating and cooling.
Characteristics and properties of cast iron
Cast iron has the following properties:
- Physical properties : specific gravity, actual shrinkage, linear expansion coefficient. For example, the carbon content of cast iron directly affects its specific gravity.
- Thermal properties . Thermal conductivity is usually calculated using the displacement rule. For the solid state of a metal, the volumetric heat capacity is 1 cal/cm3*оС. If the metal is in a liquid state, then it is approximately equal to 1.5 cal/cm3*oC.
- Mechanical properties . It is noteworthy that these properties are influenced both by the base itself and by the shape and size of the graphite. Gray cast iron with a pearlite base is the most durable, and with a ferritic base it is the most ductile. The lamellar form of graphite is characterized by the maximum reduction in strength, while the spherical form has the minimum reduction.
- Hydrodynamic properties . The presence of manganese and sulfur in the composition affects the viscosity of the material. It also tends to increase when the temperature of the alloy passes the point at which solidification begins.
- Technological properties . This metal is characterized by excellent casting qualities, as well as resistance to wear and vibration.
- Chemical properties . As the electrode potential decreases, the structural components of the alloy are arranged in the following order: cementite - phosphide eutectic - ferrite.
The properties of the alloy are also influenced by special impurities:
- The addition of sulfur significantly reduces fluidity and reduces refractoriness.
- Phosphorus allows you to make products of various shapes, but at the same time reduces its strength.
- The addition of silicon reduces the melting point of the material and also significantly improves casting properties. Silicon content in different percentages makes it possible to obtain alloys of different colors: from ferritic to pure white.
- The presence of manganese in the alloy significantly increases the hardness and strength of the material, but at the same time its casting and technological qualities deteriorate.
- In addition to these impurities, the alloy may also contain other components. In this case, the materials are called alloyed. Most often, titanium, aluminum, chromium, copper and nickel are mixed with cast iron.
Marking of white cast iron
To mark white cast iron, letters of the Russian alphabet and numbers are used. If it contains impurities, then the marking begins with the letter “H”. The composition of the available alloying additives can be determined by the following letters P, PL, PF, PVK. They indicate the presence of silicon. If the resulting metal has increased wear resistance, then its marking will begin with the letter “I”, for example ICHH, ICH. For example, the presence of the designation “Ш” in the marking means that the alloy structure contains spherical graphite.
The numbers indicate the amount of additional substances present in white cast iron.
Brand CHN20D2ХШ is deciphered as follows. This is a heat-resistant high-alloy metal. It contains the following elements: nickel - 20%, copper - 2%, chromium - 1%. The remaining elements are iron, carbon, and spherical graphite.
Application area
This alloy is used in the following industries: mechanical engineering, machine tool building, shipbuilding. Some elements of household products are produced from it. In mechanical engineering, it is used to make parts for trucks and cars, tractors, combines and other agricultural machinery. The use of alloying additives makes it possible to obtain specially specified properties. For example, they are used in the manufacture of slabs with different surface shapes.
White iron casting
Bleached cast iron has a fairly limited scope. It is used to produce parts of simple configurations. For example: balls for mills, wheels for various purposes, parts for rolling mills.
It is widely used in the production of parts for such large units as hydraulic and molding machines, and other industrial mechanisms in this area. A specific feature of their work is that they are constantly exposed to abrasive material.
Source
Types of cast iron according to carbon state
Since the state of carbon in the melt changes under different cooling conditions and in the presence of other substances in the alloy, the properties of cast iron can also differ significantly. This can be seen with the naked eye by the color of a fresh, rust-free fracture. As a rule, darker cast irons (gray cast irons) contain the largest amount of free carbon in the form of various forms of graphite inclusions.
The content of chemically bound carbon is reflected in the color of the alloy; it is noticeably lighter; such cast iron is called white. Pearlitic cast iron can contain equal amounts of bound and free carbon, which is also reflected in the color of the products. For several centuries, there has been a simplified classification of cast iron by color, dividing cast iron into three types: white, gray and half.
White cast iron
White cast iron has the lightest fracture. The largest amount of carbon in it is associated with iron and other metals. Such an alloy is formed during rapid cooling. The use of this type of alloy is limited due to its low strength.
As a rule, white cast iron is processed for the production of other types of cast iron and steel.
Gray cast iron
With slower cooling, significantly more processes take place in the melt. Carbon partially binds to metals, but a significant part of it crystallizes in its pure form, forming graphite inclusions . The size and shape of these inclusions depend on the cooling rate of the melt, as well as on the presence of various impurities in it. This is how cast iron is obtained, products from which are in demand in various sectors of the economy and in everyday life. Among the varieties of this type, a distinction is made between malleable and high-strength cast iron.
Half cast iron
Under special cooling conditions, alloys are obtained in which the amount of bound and free carbon is approximately equal. Machine parts are made from it, the main requirement for which is wear resistance due to friction: crankshafts, wheel pairs and much more. In this case, graphite plays the role of a lubricant.
Which cast iron is called eutectic
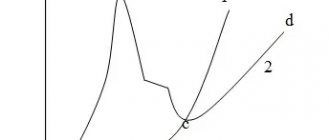
The structure of iron-carbon alloys is analyzed using the Fe–Fe3C phase diagram (Fig. l).
Show Figure 1
Due to the presence of a large amount of cementite, white cast iron has high hardness (450–550 HB), is brittle and practically cannot be cut. Therefore, white cast iron has limited use as a structural material.
A common structural component of white cast iron is ledeburite. Ledeburite is a mixture of austenite and cementite formed by a eutectic reaction when a liquid of composition point C (4.3% carbon) is supercooled below a temperature of 1147 °C:
Cast iron containing 4.3% carbon (point C) is called white eutectic cast iron. To the left of point C are hypoeutectic, and to the right are hypereutectic white cast irons.
In hypoeutectic white cast irons, austenite crystallizes from the liquid phase, then the eutectic, ledeburite.
When cast iron is cooled in the temperature range from 1147 °C to 727 °C, austenite is depleted in carbon, its composition changes along the ES line, and secondary cementite CII is released. With slight supercooling below 727 °C, austenite of point S composition decomposes into pearlite P(F + C) by eutectoid reaction.
Secondary cementite, released along the boundaries of austenite grains, merges with ledeburite cementite, so under a microscope it is difficult to distinguish inclusions of secondary cementite.
Thus, at room temperature, hypoeutectic white cast iron contains three structural components - pearlite, secondary cementite and converted ledeburite (Fig. 2).
Rice. 2. Microstructure of white cast iron (schematic representation on the left): a) hypoeutectic; b) eutectic; c) hypereutectic
The converted ledeburite, in turn, consists of pearlite and cementite.
Eutectic white cast iron at room temperature consists of one structural component - converted ledeburite.
In hypereutectic white cast iron, primary cementite CI crystallizes from the liquid in the form of flat needles, then ledeburite is formed.
At room temperature, hypereutectic white cast iron contains two structural components: primary cementite and converted ledeburite.
The phase composition of white cast irons at room temperature is the same as in carbon steels in equilibrium; they all consist of ferrite and cementite.
Maritime State University named after Admiral G.I. Nevelsky. Department of Materials Technology
Source
Cast iron production technologies
As you know, cast iron is produced in special blast furnaces. The main raw material for its production is iron ore. The manufacturing process consists of the reduction of iron ore oxides and the resulting production of another material - cast iron. For its production, fuels such as coke, thermoanthracite, and natural gas are used.
To produce one ton of pig iron, about 550 kilograms of coke and approximately a ton of water are required. The volume of ore loaded into the furnace will depend on the iron content in it. As a rule, ore is used, which contains at least 70% iron. The thing is that it is not economically feasible to use a lower concentration.
The first stage in the production of cast iron is its smelting. Ore is poured into the blast furnace, and then coking coal, which is necessary to pump and maintain the required temperature inside the furnace shaft. During combustion, these components take an active part in the ongoing chemical reactions as iron reducers.
Meanwhile, flux is immersed in the furnace, which acts as a catalyst. By accelerating the melting of rocks, it thereby supports the rapid release of iron. It is important to know that before loading into the furnace, the ore undergoes the necessary pre-treatment. It is crushed in a crushing plant because smaller particles melt faster. It is then washed to remove non-metal particles. Next, the raw material is fired, as a result of which sulfur and other foreign components are extracted from it.
At the second stage of production, natural gas is supplied through special burners into the filled and ready-to-use furnace. Coke is involved in heating the raw materials. Carbon is released, which combines with oxygen to form an oxide. It, in turn, promotes the recovery of iron from ore.
As the volume of gas in the furnace increases, the rate of the chemical reaction decreases. It may even stop completely when a certain gas ratio is reached. Carbon penetrates the alloy and combines with iron to form cast iron. Unmelted elements remain on the surface and are soon removed. Such waste is called slag. It is used to make other materials.
eutectic cast iron
Useful
See what “eutectic cast iron” is in other dictionaries:
Cast iron with vermicular graphite is cast iron with improved mechanical properties compared to gray cast iron, which is explained by the favorable form of vermicular graphite. Cast iron is obtained by processing a melt of rare earth metals (Ce, Y, etc.) in the form of multicomponent alloys introduced into the furnace crucible ... Encyclopedic Dictionary of Metallurgy
cast iron with vermicular graphite - cast iron with vermicular (worm-shaped) graphite. Cast iron with vermicular graphite occupies an intermediate position between cast irons with lamellar and nodular graphite, combining good casting properties ... Encyclopedic Dictionary of Metallurgy
Nodular cast iron is a high-strength cast iron in which the graphite has a spherical shape, which determines its high mechanical properties. In contrast to cast iron with flake graphite, the mechanical properties of cast iron with nodular graphite are large... ... Encyclopedic Dictionary of Metallurgy
Cast iron - [(pig) iron; hot metal] a multicomponent alloy of iron with carbon (> 2.14% C) and other elements, characterized by a eutectic transformation. Cast iron is the most common material for making castings, which is due to the optimal... ... Encyclopedic Dictionary of Metallurgy
gray cast iron - cast iron in which C is partially or completely (except for carbon in ferrite) in a structurally free state in the form of lamellar graphite; has a gray fracture. Lamellar graphite disrupts the continuity of the metal base, and therefore... ... Encyclopedic Dictionary of Metallurgy
pig iron - cast iron smelted in a blast furnace and intended (in liquid or solid state) for conversion into steel, mainly in oxygen converters and open-hearth furnaces. Pig iron is characterized by a low Si content and... ... Encyclopedic Dictionary of Metallurgy
nickel cast iron - cast iron with lamellar or nodular graphite containing from 0.3 0.7 to 19 21% Ni. Nickel cast iron is used as a non-magnetic, corrosion-resistant, heat-resistant and cold-resistant material. Nickel cast iron is successfully used... ... Encyclopedic Dictionary of Metallurgy
foundry cast iron - pig iron cleaved, containing Encyclopedic Dictionary of Metallurgy
silicon cast iron - 1. Cast iron alloyed with ≤ 20% Si. To improve the mechanical properties, silicon cast iron is sometimes alloyed with 8-10% Cu. High-silicon cast irons (> 12.0% Si) have increased shrinkage and are prone to the formation of shrinkage cavities. Silicon ... ... Encyclopedic Dictionary of Metallurgy
malleable cast iron is cast iron with flake graphite obtained by annealing white cast iron. Compact graphite ("annealing carbon") provides the high strength and ductility of malleable cast iron. The main advantages of malleable cast iron are... ... Encyclopedic Dictionary of Metallurgy
Source
Foundry gray cast iron
Foundry gray cast iron got its name due to its high casting properties (fluidity and low shrinkage), as well as due to its dark gray color. At the fracture it has a coarse-grained structure. Soft, easy to cut. The hardness of cast gray cast iron is 140 ... 260 HB. Tensile strength σв 100…450 MPa (10…45 kgf/mm2). Relative elongation δ 0.2 ... 0.5%. In the domestic mechanical engineering industry, up to 74% of all critical castings are produced from foundry gray cast iron. Based on their microstructure, gray cast irons are divided into ferrite-graphite, ferrite-pearlite and pearlite (Fig. 2). The carbon in these cast irons is in a free state in the form of graphite. The greater the mass fraction of carbon, the greater the graphite structure in gray cast iron and the lower its mechanical properties, therefore the maximum carbon content is limited to its hypoeutectic limits, i.e., no more than 4%, but practically up to 3.7%.
Reducing the carbon content reduces its castability. In this regard, a lower limit on the mass fraction of carbon is set. It is approximately 2.2%. The lower limit is accepted for thick-walled castings, the upper limit for thin-walled ones.
Blast furnace shops produce gray cast iron in the form of pigs, which are supplied to the foundries of machine-building plants.
Cast gray iron consists of iron, carbon, and other chemical elements, and therefore is not a two-component alloy. In addition to carbon, it contains silicon, manganese, sulfur and phosphorus. Silicon and manganese influence the graphitization process, microstructure formation, and mechanical and technological properties of gray iron castings.
Carbon affects the properties of cast iron depending on the form of the connection with iron, i.e., on the structure that is formed in the alloy. The formation of structures is collectively influenced by the melting and cooling conditions, as well as the presence of accompanying chemical elements: manganese, silicon and, slightly, sulfur and phosphorus. Silicon with a mass fraction of 3 ... 5% in gray cast iron promotes the release of carbon in the form of graphite. By changing the mass fraction of silicon, it is possible to obtain castings with different structures, and with changes in the structure, the mechanical properties of cast iron also change. For example, cast iron with a structure in the form of lamellar graphite has a relative elongation δ = 0.2 ... 1.1%, and cast iron with a flake-shaped graphite structure has a relative elongation δ = 5 ... 10%. Silicon promotes the formation of the microstructure of graphite, gives cast iron a number of valuable mechanical, technological and operational properties, and improves machinability. In addition, graphite inclusions (porous, soft) quickly dampen vibrations and vibrations and disperse shock loads throughout the mass of load-bearing parts. Cast iron parts are insensitive to mechanical damage. Due to the graphite structure, gray cast iron has high anti-friction properties. In this case, the graphite acts as a lubricant. Due to the listed properties, silicon is a constant and essential element in foundry gray cast irons.
Rice. 2. Microstructures of cast gray cast irons: a - ferritic-graphite; b - ferrite-pearlite; c - pearlitic
Manganese prevents the graphitization of cast iron, whitens it, promotes the formation of a crushed perlite structure (ferrite + cementite), improving mechanical properties. The mass fraction of manganese in gray cast iron ranges from 0.2 ... 1.1%, while strength, wear resistance and hardness increase. With a higher manganese content, the structure of pearlite and ferrite decreases, the structure of cementite increases, and cast iron becomes hard but brittle.
Sulfur is a harmful impurity. It has a negative effect on the mechanical and casting properties of gray cast iron, reduces fluidity, increases shrinkage, and promotes the formation of cracks. The mass fraction of sulfur for small castings is 0.08%, for large castings, which do not require increased fluidity, 0.10 ... 0.12%.
Phosphorus in foundry cast iron is a useful admixture, as it increases fluidity. In addition, phosphorus promotes the formation of a structure that increases the overall hardness and wear resistance of castings. A high phosphorus content (up to 0.7%) increases the cold resistance of cast iron, therefore, in castings operating under loads, the mass fraction of phosphorus can reach 0.3%, and in castings operating without loads (art and household castings) - 0.7 %.
Phosphorus has no effect on the formation of microstructure and graphitization. In practice, using structural diagrams, depending on the mass fraction of carbon and silicon in cast iron, its approximate microstructure in castings with a wall thickness of 50 mm is determined.
According to GOST 1412-87, there are the following grades of gray cast iron: SCh10, SCh15, SCh18, SCh20, SCh21, SCh24, SCh25, SCh30, SCh35, SCh40 and SCh45, where the letters SCh mean foundry gray cast iron, and the numbers indicate tensile strength. For example, cast iron grade SCh15 has a tensile strength of 150 MPa (15 kgf/mm2).
Thus, casting gray cast irons have high mechanical properties (σв - up to 450 MPa (45 kgf/mm2) and also high technological properties (casting properties, machinability, etc.). In addition, as already noted, casting gray cast iron has the ability dampen and dissipate vibrations and loads. This property is called the damping property. It is widely used in machine tool construction. From gray cast iron, which has a damping property, the beds of machine tools, machines and other supporting structures are cast, which allow creating the accuracy and rigidity of the machine-device-tool system - detail (AIDS).
The main technological properties are high fluidity and machinability. Gray cast iron castings lend themselves well to processing on various metal-cutting machines: turning, milling, planing, drilling, grinding and scraping. Due to the wide range of mechanical properties (strength and hardness), this cast iron is used in various sectors of the economy. For example, low-grade gray cast iron is used for the manufacture of castings that operate without loads (household and artistic castings, weights, stands, covers, plugs, plates, flanges, etc.). Cast gray cast iron with a tensile strength of 200 MPa or more is used for castings of parts operating under medium loads (pipes, frames, brackets, gearbox housings, etc.). Cast iron with a tensile strength of 300 MPa or more is used for parts operating under high loads (bearing housings, pulleys, gear and worm pairs, cylinder blocks, block heads, pistons, clutch discs, pump housings, steam turbine cylinders, crankshafts, sprockets, brake drums, etc.).