What is nickel plating?
Nickel plating is the process of applying a thin layer of nickel metal to a product to give it the necessary properties.
Coatings are widely used as a sublayer when coating with precious metals, as well as to improve electrical conductivity, increase hardness, protect in alkaline environments and give a highly decorative appearance.
Nickel is a silvery-white metal with a strong luster. The atomic mass of nickel is 58.69 g/mol, density 8.9 g/cm3. It has an electrochemical equivalent of 1.095 g/(A*h), its standard potential is -0.25 V.
Nickel coatings are easily passivated in air and under the influence of strong oxidizing agents. Thanks to this, the coating has high corrosion resistance. With a coating thickness of 125 microns, the base metal is already protected from the effects of industrial gases and solutions. In less aggressive environments, 50-100 microns are sufficient. Nickel is completely stable in alkalis and organic acids of an oxidizing nature.
| Designation | N. b - brilliant N - matte Nickel coating designation |
| Thickness | 6-50 microns (greater thickness is also possible) |
| Microhardness | 3420-6900 MPa |
| Electrical resistivity at 18°C | 7.23-10-8 Ohm⋅m. |
| Permissible operating temperature | 650oC |
| Light reflectance | up to 75% |
In a nickel-steel galvanic pair, nickel is the cathodic coating and, therefore, can only provide protection if there are no bare spots or pores. Therefore, it is necessary to obtain coatings with minimal porosity. In a nickel-copper pair, nickel is the anode.
The hardness of nickel obtained from electrolytes without organic additives, which include brightening agents, wetting agents and leveling additives, usually ranges from 3000-4000 MPa. With the introduction of additives, the hardness increases to 6000-7000 MPa. Tensile strength varies accordingly from 60 to 175 kgf/mm2.
Coatings have reduced ductility, but after annealing at 900°C their plastic properties are significantly improved.
There is information about the possibility of using coatings in equipment related to milk processing.
Figure 1 shows the microstructure of the surface of the matte coating on the product.
Figure 1 — Microstructure of the surface of a matte nickel coating.
How to increase the service life of the coating?
The resulting coating has a porous structure. Therefore, the metal of the product is susceptible to corrosion. To reduce the risk of its occurrence, the nickel layer is coated with lubricants. After applying them, the item is immersed in a container with fish oil. After 24 hours, its excess is removed using a solvent.
If the product is large in size and it is impossible to immerse it in a container, then its surface is simply rubbed with fish oil. This procedure will need to be carried out twice, with a time interval of about 12 hours. 48 hours after treatment, remaining fat must be removed.
Nickel plating protects metal from corrosion
There are two ways to carry out nickel plating of steel at home. This process is simple, but requires careful preparation and extreme care when performing. It is necessary to purchase high-quality components for preparing the solution, prepare the work area, containers, tools and devices in advance.
During work, it is important to observe safety measures: protect your eyes and skin from chemicals, ensure adequate ventilation of the room, and prevent the possibility of ignition of the mixture and the electrical installation.
Video on the topic: Chemical metallization - nickel plating
Useful articles
The process of chrome plating metal products at home
Pickling stainless steel at home
Technology for galvanizing at home
Electrolytes for application.
For nickel plating, sulfate, chloride, sulfamine, borofluoride, oxalate and other electrolytes are used, in which nickel is in the form of a divalent cation. A large number of compositions and deposition modes have been developed that make it possible to obtain nickel deposits with various physical and chemical properties.
Watts' sulfate electrolyte is most often used, since the substances it contains are the most accessible, and it is easy to prepare and maintain.
The main component of the sulfate electrolyte is nickel sulfate NiSO4•7H2O. Technical nickel sulfate grade CH-1 is green crystals. Solubility without heating reaches 300 g/l.
In addition to nickel salts, which are sources of nickel cations, the electrolyte contains components designed to increase electrical conductivity, stabilize acidity (buffer additives), improve the solubility of anodes (chlorides), add shine to deposits, and prevent various defects encountered during nickel plating.
If the concentration of NiSO4•7H2O does not exceed 300 g/l, Na2SO4•10H2O and MgSO4•7H2O are sometimes added to the electrolyte to increase electrical conductivity. Sodium sulfate has significantly greater electrical conductivity, but magnesium is included in nickel coatings, making them softer and lighter.
Boric acid is the most widely used buffer compound. Boric acid regulates the pH not only in the total volume of the electrolyte, but also in the near-cathode layer, in which the pH level continuously increases due to discharge and hydrogen release. At pH>4, precipitation occurs through the film of the resulting nickel hydroxide. For low pH electrolytes, fluoride additives are more effective.
Electrolytic nickel plating
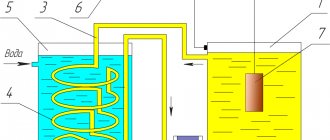
The essence of the technology of electrolytic nickel plating of metal parts, which also has another name - “galvanic nickel plating”, can be considered using the example of how copper plating of the surface of a metal product is carried out. This procedure can be carried out both with and without the use of an electrolytic solution.
The part that will be further processed in an electrolytic solution is subjected to careful processing, for which the oxide film is removed from its surface using sandpaper. Then the product to be treated is washed in warm water and treated with a soda solution, after which it is washed again with water.
Large parts are best cleaned with a sandblaster.
The nickel plating process itself is carried out in a glass container into which an aqueous solution (electrolyte) is poured. This solution contains 20% copper sulfate and 2% sulfuric acid. The workpiece, on the surface of which it is necessary to apply a thin layer of copper, is placed in an electrolyte solution between two copper anodes. To start the copper plating process, an electric current must be applied to the copper anodes and the workpiece, the value of which is calculated based on the indicator 10–15 mA per square centimeter of the part area. A thin layer of copper on the surface of the product appears after half an hour of its presence in the electrolyte solution, and such a layer will become thicker the longer the process takes place.
Installation diagram for electrolytic nickel plating
You can apply a copper layer to the surface of the product using another technology. To do this, you need to make a copper brush (you can use a stranded wire, having first removed the insulating layer from it). Such a hand-made brush must be fixed on a wooden stick, which will serve as a handle.
The product, the surface of which has been previously cleaned and degreased, is placed in a container made of dielectric material and filled with an electrolyte, which can be a saturated aqueous solution of copper sulfate. A homemade brush is connected to the positive contact of the electric current source, and the workpiece is connected to its minus. After this, the copper plating procedure begins. It consists in passing a brush, which is previously dipped in electrolyte, over the surface of the product without touching it. Using this technique, the coating can be applied in several layers, which will allow the formation of a copper layer on the surface of the product, on which there are practically no pores.
Diagram of a simple coating device
Electrolytic nickel plating is performed using a similar technology: it also uses an electrolyte solution. Just as in the case of copper plating, the workpiece is placed between two anodes, only in this case they are made of nickel. The anodes placed in the nickel plating solution are connected to the positive contact of the current source, and the product suspended between them on a metal wire is connected to the negative one.
To carry out nickel plating, including do-it-yourself, electrolytic solutions of two main types are used:
- an aqueous solution containing nickel sulfate, sodium and magnesium (14:5:3), 2% boric acid, 0.5% table salt;
- a solution based on neutral water containing 30% nickel sulfate, 4% nickel chloride, 3% boric acid.
Bright nickel plating electrolyte with the addition of organic brightening agents (sodium salts)
Bright nickel plated equalizing electrolyte. Suitable for surfaces with low cleaning class
To prepare an electrolytic solution, add one liter of neutral water to the dry mixture of the above elements and mix thoroughly. If a precipitate has formed in the resulting solution, get rid of it. Only after this the solution can be used to perform nickel plating.
Treatment with this technology usually lasts half an hour, using a current source with a voltage of 5.8–6 V. The result is a surface covered with an uneven matte gray color. To make it beautiful and shiny, you need to clean it and polish it. It should be borne in mind that this technology cannot be used for parts with high surface roughness or with narrow and deep holes. In such cases, coating the surface of a metal product with a layer of nickel should be done using chemical technology, which is also called blackening.
Electrolyte for black nickel deposition
The essence of the technological operation of blackening is that an intermediate coating is first applied to the surface of the product, the base of which can be zinc or nickel, and on the top of such a coating a layer of black nickel no more than 2 microns thick is formed. Nickel plating, made using blackening technology, looks very beautiful and provides reliable protection of the metal from the negative effects of various environmental factors.
In some cases, a metal product is simultaneously subjected to two technological operations, such as nickel plating and chrome plating.
Cathodic processes during electrodeposition.
Electrodeposition of iron group metals from solutions of simple salts has a number of features compared to other metals. The discharge of metal ions occurs at high cathodic polarization and low hydrogen overvoltage, which creates certain difficulties, since hydrogen is released at the cathode simultaneously with the metal:
Ni2+ + 2e- → Ni0
2H+ + 2e- → H2
Förster made a major contribution to the study of the kinetics of nickel electrodeposition. He and his co-workers found that the polarization curves of deposition of metals of the iron subgroup are logarithmic in nature. In this case, significant cathodic polarization is observed even at low current densities.
Initially, it was believed that nickel deposition potentials depended on the pH of the environment—as the pH decreased from 5 to 2, the potentials should have become increasingly negative.
Glaston denied these assumptions. He found that in a buffered solution of nickel sulfate, the polarization curves always slowly rise to -0.57 V, and then rise sharply. Experimentally, at 15°C, from a 1N solution of nickel sulfate, the metal begins to precipitate on the copper cathode precisely at -0.57 V. At other temperatures, the potential for nickel release shifts to a more negative region. According to Glaston, pH does not affect the potential for nickel release. When pH decreases from 5, only a shift in the sharp rise in the polarization curve (the beginning of metal release) to higher values of current density is observed. This fact turned out to be similar for iron and cobalt.
Thus, Glaston concluded that during the deposition of nickel the process is not controlled by a slow discharge. In his opinion, significant cathodic polarization is observed only before the precipitation of the metal begins; subsequently, the polarization slightly exceeds that which occurs during the deposition of lead or copper.
All of the above is illustrated in the polarization curves in Figure 2.
Figure 2 - Polarization curves in a 1N solution of nickel sulfate at different pH values. Temperature 15o C: 1 - 0.5N sulfuric acid; 2 - pH = 2.8; 3 — pH = 4; 4 — pH = 5; 5 - pH = 6.
An increase in temperature naturally reduces polarization. On the curves this is expressed in a steeper course (Figure 3).
Figure 3 - Polarization curves in a 1N solution of nickel sulfate. Temperature 95o C: 1 - pH = 2.8; 2 — pH = 4; 3 — pH = 5; 4 — pH = 6; 5 - pH = 6 (nickel-free).
It can be seen that in solutions the cathode potential, after reaching a value of -0.57 V, changes slightly further with increasing current density. At the same time, in the absence of nickel ions in the solution, no break in the polarization curve is observed. When replacing sulfates with acetates, chlorides or nitrates, the break potential of the polarization curve remains unchanged (-0.57 V at 15o C). A change in the nickel concentration in the solution by 0.01N resulted in a potential shift of -0.06V. A change in temperature to 55°C shifted the kink potential to -0.43 V, and an even greater increase in temperature (up to 95°C) shifted the kink potential to an even more positive region (up to -0.29 V). Thus, according to Glaston, at temperatures of 15o, 55o, 95o C cathodic polarization, the polarization at the initial moment of nickel release is 0.33, 0.19 and 0.06 V, respectively, and the pH value does not affect the potential of metal release. However, as the pH decreases, the current density at which this potential is reached significantly decreases (which contradicts Kolshuter's theory).
Glaston, in addition to studying polarization during nickel deposition, was the first to notice that electrolytic nickel is deposited in a very nonequilibrium form, different from metallurgical one.
Förster drew attention to the fact that Glastnom made a significant mistake - the potential values for a given current density were recorded on the basis of short-term observations, during which these potentials were not sufficiently stabilized. In addition, not enough attention was paid to protecting the cathode space from oxygen access. Having eliminated these shortcomings, Förster managed to obtain nickel at 16°C from a 1N solution of nickel chloride at a potential of -0.42 V. In sulfate solutions, nickel began to precipitate at a potential of -0.50 V, i.e. 0.07V less negative than Glaston indicated. The nature of the anion according to Förster influenced the potential for nickel release—in sulfuric acid solutions it turned out to be more negative.
Further studies carried out by Thompson in a standard nickel bath in the presence of various foreign salts showed the dependence of the course of the polarization curves on the composition of the introduced salts (Figure 4).
Figure 4 - Polarization curves in normal nickel solutions in the presence of other salts: 1 - standard bath of 1N nickel sulfate, 0.25N ammonium chloride, 0.25N boric acid; 2 - standard bath at 5o C; standard bath + 0.25N sodium fluoride; 4 - standard bath + 0.50N sodium citrate; 5 - standard bath + 1N sodium citrate; 6 - standard bath + 1N sodium tartrate; 7 - standard bath + 1N sodium acetate; 8 - standard bath + 1H NaH2PO4.
Cathode polarization has a noticeable effect on the structure of the electrolytic deposit and on the uniformity of metal distribution on the cathode surface. From this point of view, the cathodic polarization should be raised as much as possible. In some cases, without noticeable cathodic polarization, the process does not proceed at all.
Types of nickel plating
Nickel plating in simple home conditions is carried out in two ways:
- electrolytic;
- chemical
The choice of method depends on the structure and shape of the material.
Electrolytic nickel plating
The electrolytic method uses substances that partially or completely consist of ions and have ionic conductivity. Nickel coating is applied due to the electrochemical properties of these substances. The most widely used electrolytes are sodium and chromium sulfate.
Depending on the degree of reflection of the coating, nickel plating is distinguished:
Functions of electrolytic nickel plating
- matte;
- shiny.
To apply a matte finish, electrolytes without additives are used. Products with a matte finish do not have a metallic sheen.
Bright nickel plating is obtained by adding special brightening agents based on chloramine, propargyl alcohol, benzosulfamide and other oxidizing agents to the electrolyte.
The best protection of nickel coating is achieved with minimal porosity of the protective layer. For this purpose, it is copper-plated or a multilayer structure of the material is used.
For your information. With the same thickness, multilayer coatings are several times more reliable than single-layer materials.
The most common examples of multilayer materials are copper-nickel-chromium coatings.
The main disadvantages of electrolytic nickel plating are:
- high degree of porosity;
- uneven deposition of nickel;
- difficulty in processing surfaces with complex shapes.
Electroless nickel plating
The method is based on the property of nickel ions to be reduced in a liquid medium. For this purpose, sodium hypophosphite or other chemical reagents are used. The chemical method allows you to process products with complex surface shapes.
The disadvantage of this method is the relative high cost of dry reagents used to prepare aqueous chemical solutions.
Anodic processes during electrodeposition.
The mechanism of dissolution of nickel anodes is schematically expressed by the reaction equation:
Ni0 → Ni2+ + 2e-
Nickel anodes are prone to passivation. Passivation of anodes leads to a violation of the composition of the electrolyte (nickel will be produced, and acidity will increase). The passive state of the anodes is maintained by slightly soluble nickel salts formed on their surface when the permissible anodic current density is exceeded. From the moment passivation begins, the anode becomes more and more electropositive and the reaction begins:
Ni - 3ē → Ni3+
Trivalent nickel ions are hydrolyzed:
Ni3+ + 3OH- → Ni(OH)3 Ni(OH)3 → Ni2O3 + H2O
The resulting dielectric Ni2O3 promotes even deeper passivation. The passivation current density depends on the concentration of sulfate and chloride ions. Complete passivation leads to the release of oxygen and chlorine at the anode. The anodic process during nickel plating is discussed in more detail in the article.
Influence of electrolysis mode on coating quality and current efficiency.
The properties of nickel coatings are greatly influenced by:
- Bath composition;
- Temperature;
- pH;
- Current density;
- Foreign impurities.
5.1 Influence of electrolyte composition on the properties of nickel coatings.
All nickel plating electrolytes are divided into the following main groups:
- Sulfate (Watts);
- Sulfamine;
- Fluoroborate;
- Chloride;
- Fluorosilicic.
In practice, the first two are most often used. The remaining electrolytes are intended for producing matte nickel at high current densities, with 100% current output, or for coating aluminum products directly by electrolysis. All of them are much less common.
Watts' electrolyte can be used to produce both matte and shiny coatings. Currently, about 80% of all nickel coatings are bright. It is also possible to obtain shiny coatings from sulfamine electrolyte with certain additives, but more often it is used to obtain matte ductile nickel with low internal stresses for electroplating or metallization of dielectrics.
The introduction of shine-forming and leveling additives into the electrolyte allows you to obtain smooth and shiny coatings straight from the bath. Bright nickel plating has a number of advantages over matte nickel:
- the labor-intensive operation of mechanical polishing is eliminated;
- metal consumption is reduced due to mechanical polishing on corners, edges and edges;
- the number of technological operations is reduced and conditions are created for automation of the entire technological cycle;
- the deposition process is intensified due to the use of higher current densities.
The main disadvantages of shiny coatings compared to matte ones are strong hydrogenation, the presence of increased internal stresses and a large number of impurities that impair mechanical properties.
The current efficiency in a sulfate electrolyte increases with increasing concentration of nickel ions in the electrolyte.
The effect of chloride concentration on the physical and mechanical properties of nickel coatings is shown in Figure 5.
Figure 5 - Effect of chloride concentration on elongation, internal stress, hardness and tensile strength of electrodeposited nickel from Watts electrolyte solutions at pH=3.0, temperature 55°C and current density 5A/dm2.
Let us take a closer look at the effect of additives in the nickel plating electrolyte. All additives to the nickel plating electrolyte are divided into shine-forming, anti-pitting (wetting), leveling, and electrically conductive. They can be of both organic and inorganic origin. Many additives are not used today as they are obsolete (phthalimide, formalin, chloramine B, etc.).
5.1.1 Brightening agents in nickel plating electrolyte.
According to one of the accepted classifications, shine-forming additives are divided into two classes:
- Weak shine formers (class I)
make it possible to obtain shiny coatings only on a polished surface; their shine is inversely proportional to thickness. They do not affect cathodic polarization. These include methenamine, saccharin, chloramine B, disodium salt of naphthalene-1,5-disulfonic acid and others. - Strong shine formers (II class)
help to obtain shine not only on polished, but also on matte surfaces, and the shine does not depend on the thickness of the coatings. They increase cathodic polarization and level out the microrelief, but worsen the mechanical properties of deposits. These include coumarin, thiourea, 1,4-butynediol and others.
It has been established that brightening agents of the second class, especially those with double and triple bonds, are, as a rule, hydrogenated during electrolysis, and the sulfo groups of brightening agents of the first class are ultimately reduced to sulfide. Nickel sulfide, when included in the sediment, deactivates the catalytic centers of nickel, slows down the parallel reaction of the discharge of hydrogen ions and the hydrogenation processes of brighteners, and reduces the hydrogenation of sediments. This explains the advantageous use of both strong and weak shine formers.
Currently, among the brighteners, butynediol-saccharin binders are most often used due to the fact that their behavior has been best studied, and methods for purifying the electrolyte from the decomposition products of these additives have been developed.
5.1.2 Leveling additives.
Leveling additives, being surfactants, block protruding parts of the surface, and therefore deposition occurs in microcavities. Leveling additives include, for example, NIB-3. Some strong shiners also have a leveling effect.
5.1.3 Wetting agents.
Nickel plating is characterized by a phenomenon called pitting. Bubbles of hydrogen gas are retained on the cathode surface and in these places further discharge of nickel becomes impossible. Nickel begins to discharge near the bubbles (Figure 6). Pores appear on the coating, and it loses its protective and decorative properties. The adhesion of bubbles to the cathode is facilitated by all substances that increase surface tension. Hydroxides and organic compounds, as well as their decomposition products and even dust, can have a strong impact.
Figure 6 - Scheme of pitting formation on a nickel coating.
Wetting additives help reduce the surface voltage of the electrolyte and remove dirt and hydrogen bubbles from the cathode surface. Wetting additives include isoprylnaphthalene sulfonic acid, sodium lauryl sulfate, Progress detergent, etc.
It should be noted that most wetting and shine-forming additives are sulfo compounds. During electrodeposition, nickel sulfamide is formed as a result of a series of transformations. The sulfur content in sediments in large quantities adversely affects the mechanical and corrosion properties of the latter.
5.1.4 Electrically conductive additives.
Mainly to increase the electrical conductivity of the solution and, accordingly, reduce the voltage on the bath and save electricity, inorganic salts are introduced into it. Most often it is sodium, potassium or magnesium sulfate. However, as will be said in paragraph 2.6, these additives contribute to alkalization of the near-cathode layer and deterioration of the quality of the coating.
5.2 Influence of impurities in the electrolyte on the quality of nickel coating.
Nickel deposits are very sensitive to impurities entering the electrolyte. These impurities are divided into inorganic and organic.
Inorganic impurities cause the formation of brittle, cracking deposits. In the presence of copper, the deposits become rough. Zinc impurities cause dark and even black streaks to appear. In the presence of even small impurities of lead, a dark, scaly coating is formed that easily crumbles. Of the anions, the most harmful is NO3-, which promotes peeling of coatings. Such electrolytes are unsuitable for use. Chromic acids have a similar effect.
Organic compounds cause pitting, brittleness and roughness of coatings. If the electrolyte is contaminated with organic matter, it is impossible to obtain shiny precipitates.
5.3 The influence of temperature and stirring of the nickel plating electrolyte on the quality of coatings.
As the temperature increases, the current efficiency of nickel increases, since due to the acceleration of the diffusion process, polarization decreases - the nickel deposition potential becomes more positive. In this case, the hydrogen overvoltage changes slightly. Stirring the solution has the same effect.
The effect of electrolysis temperature on the physical and mechanical properties of nickel coatings is shown in Figure 7.
Figure 7 - Dependence of elongation, tensile strength and hardness of nickel coatings on electrolyte temperature at pH = 3.0 and current density 5 A/dm2.
5.4 Influence of electrolyte pH on the quality of coatings.
Nickel ions in the electrolyte are surrounded by a shell of dipole water molecules. In the electric double layer, some of the water molecules come off. Dehydration of the last water molecules requires energy, which is manifested by an increase in overvoltage, called chemical polarization. In this case, the equilibrium potential of nickel even at low current densities becomes negative.
At low pH values <1-2, nickel is almost not deposited and only hydrogen is released at the cathode. As the pH increases, the potential for hydrogen evolution becomes more negative and conditions are created at the cathode for the joint evolution of hydrogen and nickel. In this case, the higher the pH, the lower the proportion of hydrogen evolution. At high pH values, nickel precipitation cannot be carried out, since hydrolysis begins. Hydrolysis products (nickel oxide and hydroxide), penetrating into the coating, help retain hydrogen bubbles on the cathode surface, so the deposited nickel becomes porous, rough and dark. At very high pH values, a green precipitate of insoluble nickel salts can be seen on the parts with the naked eye.
Solid, stressed sediments are formed at pH >5.5, especially at temperatures below 20 °C. An increase in temperature leads to a slight decrease in internal stresses. Sediments obtained at low pH values are softer and more elastic.
The effect of pH on the physical and mechanical properties of nickel coatings is shown in Figure 8.
Figure 8 - Effect of pH on internal stress, tensile strength, ductility and hardness of nickel electrodeposited from Watts electrolyte at a temperature of 55° C and a current density of 5A/dm2.
5.5 Influence of current density and its shape on the quality of coatings.
Increasing the current density within specified limits helps reduce internal stresses and increase the gloss of coatings. The higher the temperature, the wider the operating range of current densities. It should be remembered that when the current density is significantly increased, the coating changes from fine-crystalline (compact) to dendritic (powdery) due to a violation of the stability of the flat growth front of the deposit.
The effect of current density on the physical and mechanical properties of nickel coatings is shown in Figure 9.
Figure 9 - Effect of current density on internal stress and hardness of nickel electrodeposited from Watts electrolyte at pH=3.0 and temperature 55° C.
Promising today is the use of non-stationary modes of electrolysis and ultrasound. Nickel plating with current reversal and ultrasound exposure is carried out in sulfate electrolytes. Nickel can be passivated by anodic polarization, so deposition is carried out with short anodic pulses or intermittent current. This results in the activation of nickel during the plating process. Reversing and interrupting the current ensures the production of coatings with low porosity, low internal stresses and high protective properties.
The sequential use of asymmetrical and direct current has a positive effect. Direct current deposition can improve the shine and hardness of nickel coatings.
Electrodeposition of nickel by pulsed current makes it possible to obtain mirror-shiny coatings from conventional nickel plating electrolytes without the use of shine-forming additives.
The application of ultrasound, as well as the combined use of ultrasound and reverse current, can significantly intensify the electrodeposition process. At the same time, the permissible deposition current density increases, electrolytes of conventional composition produce light, durable and practically pore-free deposits with very small coating thicknesses, at the same time the gloss of the coatings improves, and internal stresses are reduced.
To use non-stationary electrolysis modes, it is necessary to use rectifiers with special functions.
5.6 Comparison of physical and mechanical properties of nickel coating layers obtained under different conditions from Watts electrolyte and sulfamate solution.
Figure 10 - Schematic illustration of the influence of the above-described parameters of nickel deposition from Watts electrolyte and sulfamate solution on the physical and mechanical properties of the coating.
Nickel plating at home: technology and methods of nickel plating
Nickel plating process
The nickel plating procedure involves applying a nickel coating to the surface of the product, which, as a rule, has a layer thickness of 1-50 microns . Nickel coatings can be matte black or shiny, but regardless of this, they create reliable and durable protection of the metal from aggressive influences (alkali, acid) and at high temperatures.
Before nickel plating, the product must be prepared. Preparation stages:
- the part is treated with sandpaper to remove the oxide film;
- brushed;
- washed under water;
- degrease in a warm soda solution;
- washed again.
Nickel coatings can lose their original shine over time, so very often the nickel layer is coated with a more durable layer of chromium.
Nickel applied to steel is a cathodic coating that protects the metal only mechanically.
The weak density of the protective layer contributes to the appearance of corrosion pores, where the steel part is the soluble electrode.
As a result, corrosion occurs under the coating, it destroys the steel substrate and causes peeling of the nickel layer. To prevent this, the metal must always be treated with a thick layer of nickel.
Nickel coatings are applied to:
- copper;
- iron;
- titanium;
- tungsten and other metals.
Metals such as:
- cadmium;
- lead;
- lead;
- tin;
- bismuth;
- antimony
When nickel plating steel parts, it is necessary to make an underlayer of copper.
Nickel coatings are used in various industries for special, decorative and protective purposes, and are also used as a sublayer.
The nickel plating technique is used to restore worn parts and spare parts for cars, coatings of medical instruments, chemical equipment, household items, measuring instruments, parts that are subject to light loads under the action of strong alkalis or dry friction.
Types of nickel plating
In practice, there are two types of nickel plating:
- Chemical;
- Electrolytic.
The first option is slightly more expensive than the electrolytic one, but it can provide the opportunity to create a uniform coating in thickness and quality on any areas of the product if the conditions for the solution to be accessible to them are created.
Electrolytic nickel plating at home
do you need to prepare an electrolyte at home ? Requires 3.5 g. Nickel chloride, 30 g. nickel sulfate and 3 gr. boric acid per 100 ml. water, pour this electrolyte into a container. Nickel plating of copper or steel will require nickel anodes, which must be immersed in an electrolyte.
The part is suspended on a wire between nickel electrodes. The wires that come from the nickel plates need to be connected together. The parts are connected to the negative pole of the voltage source, and the wires are connected to the positive pole. Afterwards, you need to connect a rheostat to the circuit and a milliammeter to regulate the voltage. You will need a DC source with a voltage of no more than 6 Volts.
Influence of operating conditions of coatings on their properties.
Figure 11 — Effect of operating temperature on tensile strength, yield strength and elongation of electrodeposited nickel.
Figure 12 - Effect of deposit thickness and internal stresses on the 100 million cycle fatigue life of nickel-plated AISI 4340 stainless steel.