4.2
Average rating: 4.2
Total ratings received: 261.
4.2
Average rating: 4.2
Total ratings received: 261.
The physical properties of metals distinguish them from non-metals. All metals, except mercury, are solid crystalline substances that are reducing agents in redox reactions.
Position in the periodic table
Metals occupy groups I-II and secondary subgroups of groups III-VIII. Metallic properties, i.e. the ability to donate valence electrons or be oxidized increases from top to bottom as the number of energy levels increases. From left to right, metallic properties weaken, so the most active metals are in groups I-II, the main subgroups. These are alkali and alkaline earth metals.
The degree of activity of metals can be determined by the electrochemical series of voltages. Metals that come before hydrogen are the most active. After hydrogen come weakly active metals that do not react with most substances.
Rice. 1. Electrochemical series of metal voltages.
Structure
Regardless of activity, all metals have a common structure. Atoms in a simple metal are not arranged chaotically, as in amorphous substances, but in an orderly manner - in the form of a crystal lattice. A metal bond holds the atoms in one position.
This type of connection is carried out due to positively charged ions located at the nodes of a crystal cell (lattice unit), and negatively charged free electrons, which form the so-called electron gas. Electrons separated from atoms, turning them into ions, and began to move randomly in the lattice, holding the ions together. Without electrons, the lattice would disintegrate due to the rejection of equally charged ions.
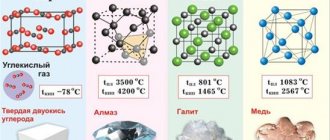
There are three types of crystal lattice. Body-centered cubic consists of 9 ions and is characteristic of chromium, iron, and tungsten. Face-centered cubic contains 14 ions and is characteristic of lead, aluminum, and silver. A hexagonal close-packed lattice of zinc, titanium, and magnesium consists of 17 ions.
Rice. 2. Types of crystal lattices.
Physics Glossary
|
Strength of solids in a broad sense
— the ability of solids to resist destruction (separation into parts), as well as irreversible changes in shape (plastic deformation) under the influence of external loads.
Strength of solids in the narrow sense
- resistance to destruction. Depending on the material, the type of stress state (tension, compression, bending, etc.) and operating conditions (temperature, load duration, etc.), various measures of the strength of solids (yield strength, tensile strength, fatigue limit) are used in technology etc.).
The destruction of a solid body is a complex process that depends on many factors, therefore the values that determine the strength of solid bodies are conditional.
Rice. 1. Dependence of the force of interaction between two atoms on the distance between them.
Physical nature of strength
The strength of solids is ultimately determined by the interaction forces between the atoms or ions that make up the body. For example, the strength of interaction between two neighboring atoms (if we neglect the influence of surrounding atoms) depends only on the distance between them (Fig. 1). At an equilibrium distance r0 ~ 0.1 nm (1), this force is zero. At smaller distances the force is positive and the atoms repel, at larger distances they attract. On critical distance force of attraction in abs. the value is maximum and equal to FT. For example, if when stretching a cylinder-rich. rod with cross section S0, the acting force P directed along its axis is such that the external force per given pair of atoms. force exceeds max. force of attraction FT, then the atoms move away from each other without hindrance. However, for a body to collapse along a certain surface, it is necessary that all pairs of atoms located on both sides of the surface in question experience a force exceeding FT. The voltage corresponding to the force F t is called. theoretical tensile strength s t (sT0.1 E, where E is Young’s modulus). However, in practice, destruction is observed under load P*, which corresponds to a voltage s = P*/S 100-1000 times less than s t· Discrepancy between the theoretical. P. t. t. with real is explained by inhomogeneities in the structure of the body (grain boundaries in polycrystalline material, foreign inclusions, etc.), due to which the load P is distributed unevenly over the cross section of the body.
Mechanism of destruction
If on a surface area of small dimensions (but significantly larger than the cross section of one atom) the local stress is greater than s t, a rupture will occur along this area. The edges of the rupture will diverge to a distance greater than rк, at which point the interatomic forces are already small, and a microcrack will form (Fig. 2). The nucleation of microcracks at stress below st is promoted by thermal. fluctuations.
Rice. 2. Griffith crack; The area in which stress is relieved is shaded. Arrows indicate the direction of voltage.
Local stresses are especially high at the edge of the formed crack, where stress concentration occurs, and the larger the crack size, the greater they are. If this size is more than a certain critical. rс, the atoms at the edge of the crack are subject to a stress exceeding st, and the crack grows further along the entire cross-section of the body at high speed - destruction occurs. The value of rc is determined from the condition that the elastic energy of the material released during crack growth covers the energy costs for the formation of a new crack surface: (where g is the energy per unit surface of the material). Before the increasing ext. the force will reach the value necessary for destruction, dep. groups of atoms, especially those that are part of defects in crystals, usually experience rearrangements, during which local stresses decrease (“relax”). As a result, an irreversible change in the shape of the body occurs - plasticity. deformation; it is also facilitated by thermal. fluctuations. Fracture is always preceded by greater or lesser plasticity. deformation. Therefore, when estimating rc, the energy g must include the work of the plastic. deformation ur. If plastic the deformation is large not only near the fracture surface, but also in the volume of the body, then the fracture is viscous. Destruction without noticeable traces of plastic. deformations called fragile. The nature of destruction is manifested in the structure of the fracture surface. In crystalline In bodies, brittle fracture corresponds to crystallographic chipping. cleavage planes, viscous - merging of microvoids and sliding. At low temperatures, destruction is predominant. brittle, at high - viscous. The rate of transition from ductile to brittle fracture is called. critical cold brittleness temperature.
Since fracture is the process of nucleation and growth of cracks and pores, it is characterized by the speed or time from the moment the load is applied to the moment of rupture, i.e., the durability of the material. Research of many crystalline and amorphous bodies showed that in a wide range of temperatures T and stresses s applied to the sample, tensile durability is determined by the relation
where approx. equal to the period of thermal vibrations of atoms in a solid (10-12 s), the energy U0 is close to the sublimation energy of the material, activation. volume V is usually several. thousand atomic volumes and depends on the structure of the material formed during the preliminary thermal process. and mechanical processing and during loading. At low temp-pax, the durability drops very sharply with increasing stress, so that for any value important for practice, there is an almost constant limiting value of stress above which the sample is destroyed almost instantly, and below it it lives indefinitely. This value of s0 can be considered the strength limit (table).
Tensile strength values
kgf/mm2 (1 kgf/mm2=10 MN/m2)
| s0 | s0/E | |
| Graphite (filamentary crystal) | 2400 | 0,024 |
| Sapphire (threaded crystal) | 1500 | 0,028 |
| Iron (whisker) | 1300 | 0,044 |
| High Carbon Steel Drawn Wire | 420 | 0,02 |
| Tungsten drawn wire | 380 | 0,009 |
| Fiberglass | 360 | 0,035 |
| Mild steel | 60 | 0,003 |
| Nylon | 50 |
Time is spent waiting for thermal fluctuations. the initiation of microcracks and their growth to critical. size . When a stress s is applied to a sample, it deforms first elastically, then plastically, near the structural inhomogeneities that were present in the initial state or that arose during plasticity. deformation, large local stresses are formed (for example, in crystals - as a result of the accumulation of dislocations). Microcracks originate in these places. Their concentration can be very high (for example, in some polymers there are up to 1015 cracks per 1 cm3). However, their sizes, determined by the scale of structural heterogeneities, are significantly smaller. Under the post. under stress, the size and concentration of cracks grow slowly and the body does not collapse until by chance (for example, as a result of the sequential merging of closely spaced adjacent cracks) one of them grows to critical. size. Therefore, when creating durable materials, care should be taken not so much to ensure that cracks do not appear, but rather to ensure that they do not grow.
Random distribution of structural inhomogeneities over the volume of the sample, in size and in degree of strength and the random nature of the term. Fluctuations lead to a scatter in the durability values (as well as the P.T.T. limit) when testing identical samples at given values of T. The greater the probability of encountering a “weak” point in a sample, the larger its volume. Therefore, the PT (breaking stress) of small samples (for example, thin threads) is higher than that of large ones made of the same material (the so-called scale effect). Areas with increased stress, where microcracks nucleate more easily, are more common near the surface (protrusions, scratches). Therefore, surface polishing and protective coatings increase P. t. t. On the contrary, in aggressive environments P. t. t. is reduced.
Properties
The structure of the crystal lattice determines the basic physical and chemical properties of metals. Metals shine, melt, and conduct heat and electricity. Industry and metallurgy have found application for the physical properties of metals in the manufacture of parts, foil, machine bodies, mirrors, household and industrial chemicals. The characteristics of metals and their uses are presented in the table of physical properties of metals.
| Properties | Peculiarities | Examples | Application |
| Metallic shine | Ability to reflect sunlight | The most shiny metals are Hg, Ag, Pd | Making mirrors |
| Density | Light - have a density of less than 5 g/cm3 | Na, K, Ba, Mg, Al. The lightest metal is lithium with a density of 0.533 g/cm3 | Manufacturing of cladding and aircraft parts |
| Heavy - have a density greater than 5 g/cm3 | Sn, Fe, Zn, Au, Pb, Hg. The heaviest is osmium with a density of 22.5 g/cm3 | Use in alloys | |
| Plastic | Ability to change shape without destruction (can be rolled into thin foil) | The most ductile are Au, Cu, Ag. Brittle – Zn, Sn, Bi, Mn | Forming, pipe bending, wire making |
| Hardness | Soft - cut with a knife | Na, K, In | Making soap, glass, fertilizers |
| Hard – comparable in hardness to diamond | The hardest is chrome, it cuts glass. | Manufacturing of load-bearing structures | |
| Melting temperature | Low-melting - melting point below 1000°C | Hg (38.9°C), Ga (29.78°C), Cs (28.5°C), Zn (419.5°C) | Production of radio equipment, tinplate |
| Refractory – melting point above 1000°C | Cr (1890°С), Mo (2620°С), V (1900°С). The most refractory is tungsten (3420°C) | Manufacturing of incandescent lamps | |
| Thermal conductivity | Ability to transfer heat to other bodies | Ag, Cu, Au, Al conduct current and heat best | Cooking in metal containers |
| Electrical conductivity | The ability to conduct electric current due to free electrons | Transmission of electricity through wires |
Rice. 3. Examples of the use of metals.
The hardest metal in the world
Our world is full of amazing facts that are interesting to many people. The properties of various metals are no exception. Among these elements, of which there are 94 in the world, there are the most ductile and malleable, and there are also those with high electrical conductivity or a high resistance coefficient. This article will talk about the hardest metals, as well as their unique properties.
Iridium ranks first in the list of metals that are distinguished by the greatest hardness. It was discovered at the beginning of the 19th century by the English chemist Smithson Tennant. Iridium has the following physical properties:
- has a silvery-white color;
- its melting point is 2466 °C;
- boiling point – 4428 oC;
- resistance – 5.3·10−8Ohm·m.
Because iridium is the hardest metal on the planet, it is difficult to process. But it is still used in various industrial fields. For example, it is used to make small balls that are used in pen nibs. Iridium is used to make components for space rockets, some parts for cars, and more.
Iridium
Very little iridium occurs in nature. Findings of this metal are a kind of evidence that meteorites fell in the place where it was discovered. These cosmic bodies contain significant amounts of metal. Scientists believe that our planet is also rich in iridium, but its deposits are closer to the Earth's core.
Ruthenium
The second position on our list goes to ruthenium. The discovery of this inert silvery metal belongs to the Russian chemist Karl Klaus, which was made in 1844. This element belongs to the platinum group. It is a rare metal. Scientists have been able to establish that there is approximately 5 thousand tons of ruthenium on the planet. It is possible to extract approximately 18 tons of metal per year.
Ruthenium
Due to its limited quantity and high cost, ruthenium is rarely used in industry. It is used in the following cases:
- a small amount of it is added to titanium to improve corrosion properties;
- its alloy with platinum is used to make electrical contacts that are highly resistant;
- ruthenium is often used as a catalyst for chemical reactions.
Chromium
Chromium is one of the hardest metals. It was discovered in Russia in 1763 in a deposit in the Northern Urals. It has a bluish-white color, although there are cases where it is considered a black metal. Chrome cannot be called a rare metal. The following countries are rich in its deposits:
- Kazakhstan;
- Russia;
- Madagascar;
- Zimbabwe.
Chromium
There are chromium deposits in other countries as well. This metal is widely used in various branches of metallurgy, science, mechanical engineering and others.
Beryllium
The fifth position in the list of the hardest metals goes to beryllium. Its discovery belongs to the chemist Louis Nicolas Vauquelin from France, which was made in 1798. This metal has a silvery-white color.
Despite its hardness, beryllium is a brittle material, which makes it very difficult to process. It is used to create high-quality loudspeakers. It is used to create jet fuel and refractory materials.
The metal is widely used in the creation of aerospace technology and laser systems. It is also used in nuclear energy and in the manufacture of X-ray equipment.
Beryllium
What have we learned?
From the 9th grade lesson we learned about the physical properties of metals. We briefly examined the position of metals in the periodic table and the structural features of the crystal lattice. Due to their structure, metals have plasticity, hardness, the ability to melt, and conduct electric current and heat. The properties of metals are heterogeneous. There are light and heavy metals, fusible and refractory, soft and hard. Physical properties are used to make alloys, electrical wires, dishes, soap, glass, and structures of various shapes.