Such a familiar school chalk to everyone, how many funny memories it holds... Only behind its apparent simplicity hides a whole history of the development of the planet. "How can it be?" - you ask. The answer to this question is in this article. You will not only study the physical properties of chalk and its use, but also become familiar with the processes of formation of deposits of limestone sedimentary rocks in the earth's crust that shaped the modern appearance of the Earth.
Minerals and rocks of biogenic origin
Approximately 130–65 million years ago, during the Cretaceous period of the Mesozoic era, the seas of the ancient planet were filled with planktonic and benthic species of foraminifera, as well as mollusks that resembled modern oysters, scallops and nautiluses. In their external skeletons and shells, they accumulated compounds of calcium, phosphorus, magnesium and, dying, formed layers of limestone silt at the bottom of reservoirs. Under the influence of high pressure and as a result of chemical processes, deposits of limestone and chalk were formed from it. The physical properties and composition of these sedimentary rocks are very similar to each other, but they also have differences. The geological processes that occurred on Earth caused the rise of individual sections of the ocean floor and the subsidence of continental zones. What did this lead to?
About copper
Among the goods that Alexandrian merchants traded in ancient times, “copper greens” were very popular. Fashionistas used this paint to add green circles under their eyes - in those days it was considered a sign of good taste. Since ancient times, people have believed in the miraculous properties of copper and used this metal to treat many ailments. It was believed that a copper bracelet worn on a hand would bring good luck and health to its owner, normalize blood pressure, and prevent salt deposition. Many peoples still attribute healing properties to copper. Residents of Nepal, for example, consider copper a sacred metal that promotes concentration of thoughts, improves digestion and treats gastrointestinal diseases (patients are given water to drink from a glass containing several copper coins). One of the largest and most beautiful temples in Nepal is called “Copper”. There was a case when copper ore became... the culprit of the accident that the Norwegian cargo ship Anatina suffered. The holds of the ship, heading to the shores of Japan, were filled with copper concentrate. Suddenly an alarm sounded: the ship had developed a leak. It turned out that the copper contained in the concentrate formed a galvanic couple with the steel body of the Anatina, and the evaporation of sea water served as an electrolyte. The resulting galvanic current corroded the ship's hull to such an extent that holes appeared in it, into which ocean water poured.
Excursion into geology
The redistribution of the surface of the lithosphere and the water shell of the planet led to the appearance of mountain chains and ridges consisting of sedimentary rocks. These are the Alps, the Caucasus Mountains, the Himalayas, the Pyrenees. And the cliffs of Dover are completely made of pure chalk. They give the English coastline a unique look and have long served as a signal for ships to approach Foggy Albion. In Russia, unique landscapes against the backdrop of chalk rocks can be seen in the village of Storozhevoy near Voronezh. Having become familiar with the geography of the distribution of biogenic rocks, now is the time to study the physical properties of chalk in more detail.
Where is copper and its alloys used?
Within the scope of this article, it is difficult to list all the areas of application of copper and its alloys. Their unique properties are used in heavy and light industry, machine and shipbuilding, aviation, medicine - in almost all sectors of the national economy and in everyday life. Each grade has its own application, for example, conductive products, parts for household appliances and electronics are made from the MO alloy, and rolled metal products, wire, and welding electrodes are made from the M1 alloy.
Copper candlestick
The use of copper and brass in plumbing (fittings, adapters, shut-off valves) and construction (roofs, domes, drainage systems, etc.) has become possible due to its high thermal conductivity and resistance to moisture. Due to its attractive appearance, bronze is the most used material for sculpture, and coins are minted from cupronickel and nickel silver and jewelry is made. Copper pipelines are an ideal solution for countries prone to frequent earthquakes.
In electrical engineering, copper is an indispensable material for the production of wires and power cables.
What determine the characteristic features of natural compounds?
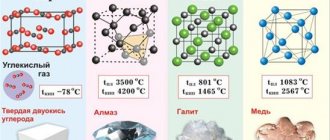
The internal spatial arrangement of atoms and molecules of the body - the crystal lattice - completely determines the state of aggregation, melting and boiling points, density, etc. These are parameters that relate to physical properties. The molecular formula CaCO3 corresponds to several crystalline compounds containing charged particles - ions - at lattice sites. These are marble, aragonite, Iceland spar, limestone and chalk. This phenomenon in chemistry is called polymorphism and is explained precisely by the shape of the crystal. Hence the conclusion follows: the physical properties of copper, gold, chalk, acetic acid and any other substance are determined by its state of aggregation, which depends on the internal structure of the compound.
Physical properties of copper
The color of copper is red, pink when broken, and greenish-blue when viewed through thin layers. The metal has a face-centered cubic lattice with parameter a = 3.6074 Å; density 8.96 g/cm3 (20 °C). Atomic radius 1.28 Å; ionic radii of Cu+ 0.98 Å; Сu2+ 0.80 Å; tmelt 1083 °C; boiling point 2600 °C; specific heat capacity (at 20 °C) 385.48 J/(kg K), i.e. 0.092 cal/(g °C). The most important and widely used properties of copper: high thermal conductivity - at 20 °C 394.279 W/(m K), that is, 0.941 cal/(cm sec °C); low electrical resistance - at 20 °C 1.68·10-8 ohm·m. Thermal coefficient of linear expansion is 17.0·10-6. The vapor pressure above copper is negligible; a pressure of 133.322 N/m2 (i.e. 1 mmHg) is achieved only at 1628 °C. Copper is diamagnetic; atomic magnetic susceptibility 5.27·10-6. The hardness of copper is 350 Mn/m2 (i.e. 35 kgf/mm2); tensile strength 220 MN/m2 (i.e. 22 kgf/mm2); relative elongation 60%, elastic modulus 132·103 MN/m2 (i.e. 13.2·103 kgf/mm2).
Types of limestones
Experts can distinguish up to 4 forms of a substance, depending on its structure and physical characteristics. Thus, pure calcium carbonate has a fine granular surface, upon contact with the surface it easily leaves a white mark and contains only up to 5% of impurities, mainly in the form of magnesium or calcium sulfate. Clay sand chalk is beige-white in color, also has a fine-grained structure, but has a higher viscosity and contains up to 10% of foreign compounds, for example, calcium sulfate, silicon or aluminum oxide. Green, yellow or gray chalk marl has even more impurities, and chalk-like limestone is easily recognized by its large yellow or white crystals with dense-cementing properties. It should be noted that in chemistry lessons, when answering the task: “characterize the physical properties of chalk,” you should focus on the first type of substance. Pure, natural calcium carbonate, containing a minimum of ballast impurities, is the substance that is offered to students as a study compound.
What is copper
Even schoolchildren can tell about copper: what substance it is, what color it is, what is made from it. In the periodic table, this chemical element is designated by the symbol Cu; it is located in the 11th group of the 4th period under No. 29. The closest neighbors are gold and silver. In English, copper is called copper, in Latin - “Cuprum”, in honor of the island of Cyprus, where large deposits of this substance were found. The origin of the Russian name is unknown.
A brief explanation of what copper is is as follows: it is a transitional soft metal of red-pink color, with atomic number 29.
How to introduce children to the properties of chalk
Students first learn about calcium carbonate in introductory chemistry lessons, which introduce the concept of pure substances and mixtures, and also discuss the basic methods of their separation. For example, during laboratory work, the teacher suggests separating metal filings and wood shavings from each other using a magnet. The sugar solution is evaporated to obtain a pure crystalline substance, and the physical properties of chalk and coal are studied after separating the two substances by settling and then filtering a suspension of calcium carbonate in water. The didactic principle of continuity and consistency in the study of new material is used when introducing students to physical phenomena and chemical reactions. The following experiment is carried out: solutions of technical soda and calcium chloride are poured into one test tube. The solution becomes cloudy and then a precipitate forms. This is chalk, it is filtered and chloride acid is added dropwise to the resulting white powder. The reaction proceeds with the rapid release of carbon dioxide bubbles. As you can see, the chemistry program, grade 8, studies the physical properties of chalk along with the main chemical feature of the substance - its ability to react with strong acids, releasing CO2.
Copper Applications
The modern market offers a wide range of consumer products containing copper: from dishes to computers. Copper is used to make coins, electrical wires, jewelry, cutlery, fittings, teapots, precision parts, artwork, musical instruments, piping and more.
For electrical conductive cables and lines, printed circuit boards and integrated circuits, electrical components (transformer windings, inductance chokes, magnetron anode bodies) only pure copper is used due to its very good electrical conductivity. Beryllium copper is used for overhead lines.
Copper is highly reflective in the infrared range and is therefore used as mirrors for carbon dioxide laser systems. Due to its good thermal conductivity, copper is often used as thermal radiators.
Copper is part of many alloys, such as golden yellow brass (with zinc ), bronze (with tin), and nickel-plated silver (with zinc and nickel ). Wrought alloys (brass and nickel-plated silver) are brought into the desired shape by plastic forming (hot forming: rolling, forging or cold forming: wire drawing, forging, cold rolling, deep drawing), while cast materials (gun steel, bronze ) is usually difficult or impossible to mold plastically.
Objects with a silvery-white (stainless steel-like) appearance are often actually high-copper alloys, since the color of the copper disappears completely when nickel is added. Modern coins are made from an alloy of copper, zinc, aluminum and tin. Copper compounds are used in color pigments, as toners, medicines and electroplating. Thanks to its noble appearance, copper is indispensable in the furniture industry and in the field of decoration.
Characteristics of calcium carbonate
The substance we are considering belongs to the group of medium salts. It is usually white in color, and, as we said, is a natural semi-rock of biogenic origin. It consists of shell particles, small quartzite crystals, magnesium and calcium carbonates, as well as oxides of these metals. Chalk absorbs and retains water, and its strength decreases. It does not dissolve in water, but forms a cloudy suspension in it. When solving experimental problems in chemistry, the physical properties of chalk, in particular its insolubility in water, are used to detect carbon dioxide. When CO2 is passed through lime water, it becomes cloudy due to the formation of an insoluble precipitate of calcium carbonate. This reaction is qualitative and is used in analytical chemistry.
Copper production
To produce copper from copper gravel (CuFeS2), the so-called copper stone (Cu2S with various FeS contents) with a copper content of about 70% is initially obtained. To do this, the source material is heated with the addition of coke and the iron oxides it contains, slagged with siliceous fillers. The resulting iron silicate slag floats in the melt on the surface and can be easily drained. The copper stone is further processed into raw copper ( black copper ) with a copper content of about 98%.
To do this, the melt is poured into the converter and air is blown in. In the first stage (slag blowing), the iron sulfide contained in it is fried into iron oxide, and the flaked quartz binds to the slag, which can be drained. In the second stage, two thirds of the remaining Cu2S is oxidized to Cu2O. The oxide then reacts with the remaining sulfide to form crude copper. The raw copper ( cement copper ) is then refined electrolytically.
Copper migrates as ions through the electrolyte to the cathode and is deposited there. The final copper content is 99.99% with very little admixture of other substances. The less noble metals of these impurities remain dissolved in the electrolyte; the more noble metals (including silver and gold) form an “electrolyte precipitate” and are further processed separately.
How to increase the viscosity and plasticity of limestone
What does the flooding of Cretaceous rock deposits with underground groundwater lead to? If the moisture content of the rock is insignificant - no more than 2%, then the strength of the crystals decreases. However, when CaCO3 layers are strongly wetted, for example by 25%, the compressive strength of the substance increases almost 3 times. At the same time, the physical properties of chalk, especially plasticity and viscosity, are enhanced. This greatly complicates the technology of its extraction. For this reason, the upper and drier layers of the deposits, although with a low content of pure calcium carbonate, are used to produce it on an industrial scale.
Copper in nature
According to estimates, the percentage of copper in the earth's crust is (4.7-5.5) 10−3%. It can rarely be found in its free form, only in the form of nuggets (individual specimens weigh up to 400 tons), formed by the oxidation of copper ores. In them its percentage is 98-100%. There are other copper-containing minerals, more than 200 of them. Among them are several especially rich in copper (Cu content is indicated in parentheses):
- bluish-green chrysocolla (36%);
- golden-green chalcopyrite (34%);
- green malachite (57.4%);
- indigo and copper-red bornite (55-65%);
- lead-black chalcocite (80%);
- all shades of green brochantite (56%);
- dark red cuprite (up to 89%).
In copper-bearing ores, the Cu content does not exceed 6%.
Where and how to use chalk
The largest amount of the substance is used to produce quicklime, calcium hydroxide and carbon dioxide. To do this, calcium carbonate is burned to form calcium oxide and carbon dioxide. The first substance is also called quicklime or boiling water. It is combined with water, the process releases a large amount of heat, resulting in slaked lime - an important raw material for the construction industry. In combination with sand and water, calcium hydroxide is used for plastering and fastening bricks during the construction of walls. Liming of acidified soils is a well-known and economically cheap method of reclamation work, which increases soil fertility and does not have a negative effect on the species composition of soil organisms.
In this work, the physical properties of chalk were studied and the areas of its application in industry and agriculture were considered.
Methods for obtaining copper
In nature, copper exists in compounds and in the form of nuggets. The compounds are represented by oxides, bicarbonates, sulfur and carbon dioxide complexes, as well as sulfide ores. The most common ores are copper pyrite and copper luster. The copper content in them is 1-2%. 90% of primary copper is mined using the pyrometallurgical method and 10% using the hydrometallurgical method.
1. The pyrometallurgical method includes the following processes: enrichment and roasting, smelting for matte, purging in a converter, electrolytic refining. Copper ores are enriched by flotation and oxidative roasting. The essence of the flotation method is as follows: copper particles suspended in an aqueous medium adhere to the surface of air bubbles and rise to the surface. The method allows you to obtain copper powder concentrate, which contains 10-35% copper.
Copper ores and concentrates with a significant sulfur content are subject to oxidative roasting. When heated in the presence of oxygen, sulfides are oxidized, and the amount of sulfur is reduced by almost half. Poor concentrates containing 8-25% copper are roasted. Rich concentrates containing 25-35% copper are melted without resorting to roasting.
The next stage of the pyrometallurgical method for producing copper is smelting for matte. If lump copper ore with a large amount of sulfur is used as a raw material, then smelting is carried out in shaft furnaces. And for powdered flotation concentrate, reverberatory furnaces are used. Melting occurs at a temperature of 1450 °C.
In horizontal converters with side blowing, the copper matte is blown with compressed air in order for the oxidation of sulfides and ferrum to occur. Next, the resulting oxides are converted into slag, and sulfur into oxide. The converter produces blister copper, which contains 98.4-99.4% copper, iron, sulfur, as well as small amounts of nickel, tin, silver and gold.
Blister copper is subject to fire and then electrolytic refining. Impurities are removed with gases and converted into slag. As a result of fire refining, copper is formed with a purity of up to 99.5%. And after electrolytic refining, the purity is 99.95%.
2. The hydrometallurgical method involves leaching copper with a weak solution of sulfuric acid, and then separating copper metal directly from the solution. This method is used for processing low-grade ores and does not allow for the associated extraction of precious metals along with copper.