Welding silicon-based carbide involves direct contact with water, during which a significant amount of acetylene gas and a lot of heat are released. Due to this operational characteristic, it is not so easy to ensure safe storage of this material. In order to do this, the carbide is placed in completely sealed tanks made of roofing metal. They should fit from 100 to 130 kg.
When welding with carbide, a large amount of acetylene is released, a gas that is highly flammable, so when opening such cans, care should be taken to ensure that there are no sparks or open flames nearby. Welding work using carbide dust with a particle size of 2 mm or less will not be carried out, since such dust dissolves almost instantly in water. If you add too much of this dust to the water, there is a real possibility of an explosion. By the way, a kilogram of carbide can lead to the release of almost a whole cubic meter of pure acetylene.
Carbide is widely used for gas-type welding, as well as for cutting metals. When acetylene begins to burn with oxygen, the flame temperature can reach over 3000 degrees Celsius. This allows this material to be used even when working with refractory materials.
What is needed for acetylene welding?
Gas welding requires two types of gases, the first is oxygen and the second is acetylene. The oxygen compound is necessary to promote the combustion of acetylene during welding and other types of work. Acetylene itself is a flammable substance.
Interesting materials:
Which is correct: silicone or silicone? How to properly attach a plinth to the floor? Which is correct: Christina or Christina? How to cut velvet with or against the pile? How to eat artichoke correctly? Which is correct: lemon or lemon? Which is correct: Lithuania or Republic of Lithuania? How to properly catch catfish on the river? How to properly marinate meat for cold smoking? How does it interfere?
Production method
Carbide is produced in electric furnaces by fusing (calcining) a mixture of coke and calcium oxide (quicklime) at temperatures ranging from 1900°C to 2300°C. The sharp and unpleasant garlic smell of carbide and the produced acetylene is caused by the content of impurities of calcium phosphides and sulfates in the carbide mixture.
The process is carried out in several stages:
- Limestone is fired.
- A powder mixture is created from processed lime and coke - a mixture.
- The resulting mixture is calcined in an electric arc furnace until it melts.
- The resulting carbide bars are crushed to obtain the desired fraction.
The average density of the carbide substance is 2.2 g/cm3. Depending on the impurity content, the color of carbide can be dark brown or dark gray.
The final product consists of 75-78% CaC2, the rest being lime and impurities. Carbide granules come in different sizes: 2x8; 8x15; 15×25; 25x80 mm. Larger granules produce more acetylene but increase reaction time. If granules of 8x15 and 15x25 mm decompose in 5-6 minutes, then granules of 25x80 mm require more than 10 minutes to decompose.
Safety precautions
Carbide for welding belongs to the class of explosive substances. Safe use of carbide is ensured by taking into account a number of conditions:
- Acetylene is a flammable gas, and dry carbide itself is also explosive, so there should be no open flames near the work site, even small ones such as lighters and lit cigarettes.
- It is prohibited to use carbide in granules up to 2 mm or carbide dust, as it dissolves very quickly, which leads to the release of large amounts of gas. Because of this, ultra-high pressure forms in the generator and an explosion may occur.
- It is prohibited to work with an angle grinder or an electric welding machine near gas welding operations and places where carbide drums are installed.
- The substance must be stored in a dry and sealed place, in which there are no water pipes, sewer pipes and, especially, gas equipment.
- Carbide dust upon contact causes irritation to the skin, eyes, and mucous membranes. Work with this substance must be carried out using personal protective equipment - goggles, gloves and a respirator.
- If carbide gets on your skin or eyes, rinse it with plenty of warm water and then carefully remove any remaining carbide with tweezers or a damp swab.
- When working with carbide in a workshop, all welding equipment must be placed in separate parts of the room, the ventilation system of the room must ensure the removal of flammable gases, and the room must be cleared of flammable materials.
- Acetylene generators are prohibited from being installed in basements and residential buildings.
- Before starting, the generator should be inspected for visible cracks or dents in the housing.
- During work, the generator must remain in a vertical position, the pressure gauge must be in working order and clearly visible to the welder or his assistant.
- At the end of the work, the carbide solution remaining in the generator must be completely exhausted, and the resulting lime must be disposed of.
- Reuse of wet carbide pieces is not permitted;
- Do not open the generator under pressure (during an ongoing reaction).
- Acetylene cylinders are stored and transported with special safety caps on the valves.
Compliance with these simple rules will ensure your own safety and the safety of people near the work site.
Carbide should be purchased from reputable welding equipment stores, since purchasing low-quality carbide can complicate welding work.
You can also order it in online stores. The price of carbide varies from 75 to 190 rubles per kilogram, depending on the manufacturer. If there is a need to regularly use equipment for gas welding and cutting, it is better to buy professional equipment manufactured at an industrial enterprise. The use of homemade generators can result in serious injuries and a threat to life.
Appearance and characteristics of technical calcium carbide
The composition in question was first obtained in 1862. The procedure involved the separation of calcium from lime, resulting in a pale gray composition without the characteristics characteristic of metals. As a result of the experiment, carbide was obtained, which subsequently began to be actively used in the production of various products.
At the beginning of the 20th century, calcium carbide began to be used to produce acetylene in large volumes. That is why active research began to be conducted to identify more productive technology.
The technical characteristics of the material determine its wide distribution. The appearance of the substance is characterized by a light gray color; carbides are produced in the form of stone or powder.
How to obtain carbon dioxide from acetylene?
When acetylene burns, carbon dioxide and water are formed. 2CH ≡ CH + 5O2 → 4CO2 + 2H2O Let’s place the test tube into the flame of burning acetylene. Soot settles on the test tube. With a lack of oxygen, acetylene does not have time to burn completely and releases carbon in the form of soot.
Interesting materials:
How to view disappearing messages on Instagram? How to view SMS history on MTS? How to view history in Google Chrome browser? How can I see my accumulated VTB miles? How can I view canceled flights? How can I view ownership? How to view your Gmail profile? How can I view past declarations? How to watch stories on YouTube? How to see hidden friends?
Physical properties
When choosing almost any material, you should pay most attention to physical properties. For the one under consideration they are as follows:
- The compound has a crystalline structure.
- The melting point is 2300 °C. It is worth considering that such a figure is characteristic only of the pure composition. The addition of various impurities to the composition causes the melting point to drop significantly.
Pure Calcium Carbide
It is worth considering that calcium carbide in most cases is in a solid state. In addition, the color can vary from gray to brown. The physical properties of calcium carbide determine its widespread use in a wide variety of industries.
Calcium carbide reaction with water
The raw material in question is most often used to combine with water, resulting in acetylene. The interaction of calcium carbide with water causes the appearance of gas with an unpleasant odor and a fairly large number of various impurities. Such a substance can only be obtained in its pure form through multi-stage purification.
The reaction of calcium carbide with water can be carried out experimentally. The features of such a procedure include the following points:
- A 1.5 liter bottle is used as a container.
- After filling it with water, a few pieces of crystalline material are added.
- The reaction leads to the appearance of excess pressure.
- Once the calcium carbide no longer reacts, burning paper is placed on the bottle. As a result of the interaction between calcium carbide and water, a gas is formed that explodes. During the experiment under consideration, a fiery cloud is formed.
Such an experiment is quite dangerous and must be carried out in compliance with safety precautions.
In conclusion, we note that the component in question has recently been often used to conduct a wide variety of experiments. The compound has a large number of properties that must be taken into account. The release of heat and gases becomes the reason why experiments are recommended only in industry.
Welding applications
Carbide is stored in special steel tanks with a volume of 100 or 130 liters. These tanks should only be opened when there is no fire or sparks in the vicinity using a wooden hammer and a brass chisel. Unused carbide in the jar is sealed with a waterproof lid.
Acetylene for welding is produced from carbide in a generator of stationary or mobile type and of different volumes. The average volume of acetylene generators is designed to receive from 5 to 15 liters of water and, accordingly, 2-5 kilograms of carbide. The yield of acetylene is considered slightly lower than theoretical and is taken to be 260-280 liters per kilogram of CaC2. It is recommended to use coarse carbide – 80 mm
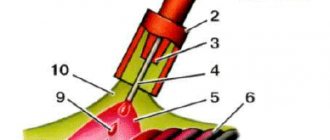
The principle of using acetylene for welding work is as follows:
- About 250 liters of acetylene are released from one kilogram of carbide, and 3-4 liters of water are required to decompose a kilogram of carbide. Knowing these proportions, the required volume of water and the amount of substance are calculated.
- In industrial generators, designed for a long and uniform process of use, carbide is dosed into the gas-forming chamber through a special hopper in automatic mode. In generators that are used for non-standardized volumes of work, the carbide is immersed in water in a special basket. The volume of acetylene produced is regulated by immersing or raising the basket.
- When the next portion of carbide is supplied and the reaction begins, the pressure in the chamber increases, which is reduced by the active release of acetylene into the burner.
- Acetylene is fed through a hose into the gas burner. The burner must be located at least 10 meters from the generator.
- The slaked lime formed during the reaction (about 1.2 kg per kilogram of carbide) is removed from the generator through a separate hopper.
In gas welding, the main advantage of using carbide is its low weight and the light weight of the equipment used. Gas cylinders for acetylene are very heavy; they must be moved on a special trolley or with 2-3 pairs of hands. The average generator weighs 15-20 kg, which allows you to move it without much effort alone or with one assistant. When moving dry carbide, it is enough to follow basic storage rules - avoid getting moisture on the substance and getting fine carbide dust on your skin and eyes.