Acetylene, formula, gas, characteristics:
Acetylene (also ethyn) is an organic substance of the alkyne class, an unsaturated hydrocarbon consisting of two carbon atoms and two hydrogen atoms.
The chemical formula of acetylene is C2H2. Structural formula of acetylene CH≡CH. Has no isomers.
The structure of the acetylene molecule:
Acetylene has a triple bond between carbon atoms.
Acetylene is a colorless gas, tasteless and odorless. However, technical acetylene contains impurities - hydrogen phosphorous, hydrogen sulfide, etc., which give it a pungent odor.
Lighter than air. Density compared to air density 0.9.
Very flammable gas. Fire and explosion hazard.
Acetylene is one of the few compounds whose combustion and explosion are possible in the absence of oxygen or other oxidizing agents.
Mixtures of acetylene with air are explosive over a very wide range of concentrations. Explosiveness is reduced when acetylene is diluted with other gases, such as nitrogen, methane or propane.
Acetylene requires great care when handling. May explode on impact, when heated to 500 °C, or when compressed above 0.2 MPa at room temperature. A stream of acetylene released into the open air can ignite from the slightest spark, including from a discharge of static electricity from a finger. To store acetylene, special cylinders filled with porous material impregnated with acetone are used. In them, acetylene is stored in the form of a solution with acetone.
Slightly soluble in water. Very soluble in acetone. It dissolves well in other organic substances (gasoline, benzene, etc.)
Acetylene has a slight toxic effect.
Safety regulations
The use of acetylene without skills and experience is prohibited. There are several rules that should be followed when working with the substance:
The acetylene content in the air in the room must be constantly monitored. To do this, you should use special automatic devices that can notify you when the gas concentration is exceeded. This figure should not be more than 0.46%.
The areas of application of acetylene are completely different, but most often it is used in welding. When working with cylinders filled with this particular gas, care should be taken. It is prohibited to place containers near open fire or near heating systems. In addition, it is prohibited to work with cylinders that are in a horizontal position, as well as if they are not secured and faulty.
When working with acetylene, use only non-sparking tools, electrical equipment and explosion-proof lighting.
If acetylene leaks from the cylinder, you should quickly close the container valve. To do this, you can use a non-sparking special key. A leak can only be determined by sound or smell.
Physical properties of acetylene:
| Parameter name: | Meaning: |
| Color | no color |
| Smell | without smell |
| Taste | no taste |
| Physical state (at 20 °C and atmospheric pressure 1 atm.) | gas |
| Density (at 20 °C and atmospheric pressure 1 atm.), kg/m3 | 1,0896 |
| Density (at 0 °C and atmospheric pressure 1 atm.), kg/m3 | 1,173 |
| Melting point , °C | -80,8 |
| Boiling point , °C | -80,55 |
| Triple point, °C | 335 |
| Auto-ignition temperature, °C | 335 |
| Self-ignition pressure, MPa | 0,14-0,16 |
| Critical temperature*, °C | 35,94 |
| Critical pressure, MPa | 6,26 |
| Explosive concentrations of gas-air mixture, % volume | from 2.1 to 100 |
| Specific heat of combustion , MJ/kg | 56,9 |
| Flame temperature, °C | 3150-3200 |
| Molar mass, g/mol | 26,038 |
* at temperatures above the critical temperature, the gas cannot be condensed at any pressure.
Welding process
The use of acetylene during welding must be carried out carefully and in accordance with certain rules. First, the burner should be purged with gas. This must be done until the smell of acetylene appears. After this, the gas is ignited. In this case, oxygen should be added until the flame becomes more stable. From the reducer at the outlet, the pressure of acetylene should be from 2 to 4 atmospheres, and oxygen - from 2 atmospheres.
Welding ferrous metals requires a neutral flame. It has a clearly defined crown and can be conditionally divided into three bright parts: the core is a bright blue color with a greenish tint, the restored flame is a pale blue hue, and the flame torch. The last two zones are working zones.
Before starting work, all parts must be cleaned and then adjusted to each other. When working with a burner, the left and right methods are also used. In the latter case, the seam cools slowly. The filler material typically moves behind the torch. With the left method, the elasticity and strength of the seam increases. In this case, the flame is directed from the welding site. Filler material should be added to the weld pool only after the torch has moved to the next position.
Chemical properties of acetylene:
The chemical properties of acetylene are similar to those of other representatives of the alkyne series. Therefore, it is characterized by the following chemical reactions:
- 1. halogenation of acetylene:
СH≡CH + Br2 → CHBr=CHBr (1,2-dibromoethene);
CHBr=CHBr + Br2 → CHBr2-CHBr2 (1,1,2,2-tetrabromoethane).
The reaction proceeds stepwise with the formation of alkane derivatives.
During this reaction, acetylene decolorizes bromine water.
- 2. hydrohalogenation of acetylene:
СH≡CH + HBr → CH2=CHBr (bromoethene).
- 3. hydration of acetylene (reaction of Mikhail Grigorievich Kucherov, 1881):
CH≡CH + H2O → [CH2=CH-OH] (enol) → CH3-CH=O (acetic aldehyde) (kat = HgSO4, Hg(NO3)2).
- 4. trimerization of acetylene (reaction of Nikolai Dmitrievich Zelinsky, 1927):
3CH≡CH → C6H6(benzene) (kat = activated carbon, to = 450-500 oC).
The acetylene trimerization reaction is a special case of the acetylene polymerization and occurs when acetylene is passed over activated carbon at a temperature of 450-500 oC.
- 5. acetylene dimerization:
СH≡CH + СH≡CH → CH2=CH-С≡CH (vinylacetylene) (kat = aqueous solution of CuCl and NH4Cl).
The acetylene dimerization reaction is a special case of the acetylene polymerization reaction.
- 6. acetylene combustion:
2СH≡CH + 5О2 → 4СО2 + 2H2О.
Acetylene burns with a white, bright flame.
- 7. oxidation of acetylene.
The course of a reaction and its products are determined by the environment in which it occurs.
- 8. acetylene reduction:
CH≡CH + H2 → C2H4 (ethylene) (kat = Ni, Pd or Pt, increased to);
CH≡CH + 2H2 → C2H6 (ethane) (kat = Ni, Pd or Pt, increased to).
What to do if there is a fire
Improper use of acetylene can lead to dire consequences. This gas explodes and causes great destruction. What to do if there is a fire?
If a fire occurs, all containers filled with acetylene should be immediately removed from the hazardous area. Those cylinders that remain should be constantly cooled with ordinary water or a special composition. The containers must cool completely.
If the gas that comes out of the cylinder ignites, the container should be closed immediately. To do this, use a non-sparking key. After this, the container must be cooled.
In the event of a severe fire, extinguishing the fire should only be done from a safe distance. In such a situation, it is worth using fire extinguishers filled with a composition containing a phlegmatizing concentration of nitrogen 70% by volume, also carbon dioxide 75% by volume, sand, jets of water, compressed nitrogen, asbestos sheet, and so on.
Application and use of acetylene:
– as a raw material in the chemical industry for the production of acetic acid, ethyl alcohol, solvents, plastics, synthetic rubbers, aromatic hydrocarbons,
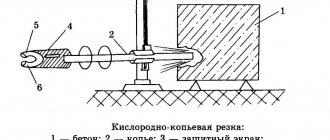
– for gas welding and metal cutting,
– to obtain carbon black,
– as a source of very bright, white light in free-standing lamps, where it is produced by the reaction of calcium carbide and water.
Receipt
In the laboratory
In the laboratory, acetylene is produced by the action of water on calcium carbide, see video of this process (F. Wöhler, 1862),
as well as during the dehydrogenation of two methane molecules at temperatures above 1400 °C:
In industry
In industry, acetylene is produced from calcium carbide and pyrolysis of hydrocarbon raw materials - methane or propane with butane. In the latter case, acetylene is produced together with ethylene. The carbide method allows one to obtain pure acetylene, but requires high power consumption. Pyrolysis is less energy intensive, but the resulting acetylene has a low concentration in the gas stream and requires separation. Economic evaluations of both methods are numerous but controversial.
Preparation by pyrolysis
Methane is converted into acetylene and hydrogen in electric arc furnaces (temperature 2000-3000°C, voltage between electrodes 1000 V). Methane heats up to 1600°C. Electricity consumption is about 13,000 kWh per 1 ton of acetylene, which is relatively high (approximately equal to the energy consumed by the carbide method) and therefore is a disadvantage of the process. The acetylene yield is 50%.
Another name is the Wolf process. First, the furnace head is heated by burning methane at 1350-1400°C. Next, methane is passed through the heated nozzle. The residence time of methane in the reaction zone is very short and amounts to a fraction of a second. The process was implemented in industry, but economically it turned out to be not as promising as it was thought at the design stage.
Methane is mixed with oxygen. Part of the raw material is burned, and the resulting heat is used to heat the rest of the raw material to 1600°C. The acetylene yield is 30-32%. The method has the advantages of a continuous process and low energy consumption. In addition, synthesis gas is also formed with acetylene. This process (Sachse process or BASF process) has received the most widespread adoption.
It is a type of oxidative pyrolysis. Part of the raw material is burned with oxygen in the furnace firebox, the gas is heated to 2000°C. Then the remainder of the raw material, preheated to 600°C, is introduced into the middle part of the furnace. Acetylene is formed. The method is characterized by greater safety and reliability of the furnace.
Pyrolysis in a low-temperature plasma jet
The process has been developed since the 1970s, but, despite its promise, has not yet been introduced into industry. The essence of the process is heating methane with ionized gas. The advantage of the method is its relatively low energy consumption (5000-7000 kWh) and high yields of acetylene (87% in argon plasma and 73% in hydrogen plasma).
Carbide method
This method has been known since the 19th century, but has not lost its importance to this day. First, calcium carbide is obtained by fusing calcium oxide and coke in electric furnaces at 2500-3000°C:
Lime is obtained from calcium carbonate:
Next, calcium carbide is treated with water:
The resulting acetylene has a high degree of purity of 99.9%. The main disadvantage of the process is the high energy consumption: 10,000-11,000 kWh per 1 ton of acetylene.