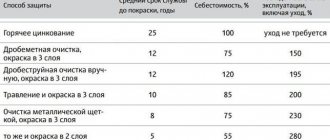
How to protect copper jewelry from oxidation. - Arts and crafts fair
New decorations have appeared in my store!
When creating them, the technique of artistic metal etching was used. Since I etched on copper, I was faced with the task of protecting my creations from the effects of the external environment, that is, from oxidation of the polished parts of the jewelry. Of course, I started surfing the Internet and this is what I found. There are many varnishes for metal: ceramic varnishes, PLASTIK 70 board varnish, Tsapon varnish, and so on. There are many pros and cons of all varnishes on different sites. Some people are generally against all varnishes, since the decoration ceases to be alive and change. I also adhere to this position, but I am faced with the problem of a damp climate. After each fair, the jewelry became patinaed on its own and had to be polished again. If you are the owner of one piece of jewelry, then polishing it takes 5 minutes, but if you are a craftsman and you have a lot of jewelry, then this becomes difficult. So, for myself, I found out one thing: there seems to be no varnish that will forever protect patinated metal from further oxidation. Because under the varnish the oxidation of the metal still continues and because of this the varnish is destroyed over time. The metal begins to darken and oxidize again.
I decided to try Tsapon varnish, since it is sold in construction and hardware stores and is generally available to the public. First you need to degrease the metal object (I used soda), then dry it and varnish it with a brush on one side, and when dry, on the other. It is advisable to coat each side 2-3 times. Under the varnish, the metal did not darken, but only became more shiny.
Metals copper and brass are susceptible to oxidation, which is why they darken. If you like jewelry made from these metals, then be prepared for this when purchasing them. You can easily restore the shine of your favorite piece of jewelry by polishing it with a jewelry cloth, and if you want a longer lasting effect, you can varnish it. Jewelry coated with varnish does not leave marks on clothes and skin, and also does not cause allergies.
I hope my little research will be useful to someone. =)
How to care for copper jewelry?
Copper items are not valued as highly as gold or silver items. Despite this, they enjoy well-deserved love among those who appreciate the unusualness and uniqueness of this material. After all, the “copper history” goes back more than 7,000 years. The metal has been used by various cultures around the world to make weapons.
Achilles himself had a copper shield forged by Hephaestus! It was used to make objects for rituals, candlesticks and dishes. Copper jewelry was worn by Egyptian and Greek fashionistas, residents of India and China. Copper provided miraculous protection on the physical and energetic levels.
Today's beauties, who know about the magical properties of metal, use copper jewelry not only to emphasize their beauty, but also as protection from the evil eye and envy.
Modern fashion
Copper jewelry is, first of all, exclusive. Almost all of them are made by hand, original and unique. In addition, the products are affordable and, unlike jewelry, can be updated frequently. Their owners know a lot about individual style and have delicate taste.
A stylish woman can always buy new jewelry to harmoniously combine with her wardrobe items. Copper earrings and a chain are perfect for both casual casual clothing, such as jeans and knitwear, and evening wear. They can look vintage depending on the treatment.
Fashionable accessories made from this material are increasingly filling women's jewelry boxes, displacing precious metals.
Health and beauty
The healing properties of copper have been known for a long time. The material cleanses and strengthens the body, removing excess water and regulating metabolism, so people with certain diseases are recommended to wear a bracelet or ring made from it. It normalizes blood pressure and has a beneficial effect on the cardiovascular system.
In addition, such products have an original, incomparable color. The magical ancient fire of the Copper Age blazes within it. Accessories will highlight the “green pool” of the eyes and the darkness of the skin. They are ideal in combination with red hair color. Elegant and luxurious.
It is worth noting that copper can cause an allergic reaction. If you are allergic to jewelry, then you will have to discard such products.
Rules of care
Unfortunately, copper products darken and oxidize during wear, which prevents them from gaining wider acceptance among the population. But do not be afraid of this, because even noble metals such as gold and silver are subject to similar changes.
Caring for copper is simple and accessible to everyone, depending on the classification of the product:
- Not patinated decorations. They have a natural appearance, are not covered with anything, and are the most beneficial for health. Products are susceptible to oxidation and darken. To clean them, use denim, carefully wiping the darkened areas until they shine.
- Patinated jewelry has an antique finish. It can be of several colors - red or brown, blue or green. The patina wears off under the influence of external factors, not worsening, but improving the appearance, giving the accessory more “antiqueness”. May stain the skin in areas of contact.
- For beauty and shine, patinated and non-patinated products are varnished. They retain an attractive appearance for a long time, do not stain the skin, and are not allergenic. Decorations coated with varnish must not be scratched or subjected to mechanical stress.
- Jewelry with stones. Regardless of the coating, they require treatment similar to other metal products with stones:
- do not drop;
- do not apply perfume or other cosmetics after putting on the accessory;
- handle the product with care;
- Do not do homework in rings, exposing them to chemicals.
It is recommended to store all copper jewelry in bags without access to air, separately in closed boxes. Polish from time to time with a hard material, do not wet, do not apply chemical liquids. Avoid exposure to sunlight and exposure to heating devices.
Popular experience says that a piece of chalk in a box with “copper riches” will avoid the process of their oxidation. Copper earrings are removed at night so as not to break or bend. It is better to store them in a box. Unlike gold and silver, copper is a soft metal and is susceptible to deformation.
What can I do to prevent the wire from darkening? | "Arabesque" Krasnodar
Do you, just like other craftsmen and needlewomen who work with wire for needlework, want to know what needs to be done to prevent the wire from darkening? In this article, we decided to answer this burning question for many, which may sound different:
- “How to cover jewelry so that it doesn’t fade over time?”
- “What varnish should I coat the wire with?”
- “What kind of wire does not darken or fade?”
- “What can I do to prevent the wire from darkening?”
How to protect copper products from plaque formation
Copper jewelry is valued not only for its beauty, but also is believed to have health benefits.
But unlike more expensive gold items, copper jewelry requires little care to avoid oxidation. When oxidized, copper rings and bracelets not only become coated, they also leave unsightly green stains on the skin. This happens when copper reacts with air and water to form copper carbonate.
Copper oxidation, although unsightly, is not harmful to health, but can be avoided with proper cleaning and daily care.
- Clean your brass thoroughly with an acidic cleaner and, if necessary, a toothbrush. There are many suitable cleaning products available in stores, but lemon juice, vinegar and even ketchup also contain enough acid for basic cleaning, and salt can be used as an abrasive to scrub away the most stubborn stains.
- Rinse the copper piece to remove any cleaning agent, then neutralize any remaining acid. Dip the item in a mixture of 1 part baking soda and 16 parts water.
- Rinse the jewelry again with clean water and dry. Lubricate all surfaces of the product with xylon or denatured alcohol. Wait until it is completely dry.
- Cover the copper piece with a thin layer of wax and polish until shiny. Jewelry wax can be purchased at a jewelry store, but car wax is also great.
- Before going to bed, be sure to remove all copper jewelry and dry it with a soft, clean cloth to remove sweat and oil. After these daily treatments, your copper jewelry will require less cleaning and polishing to maintain its best appearance.
- For longer-lasting protection, coat your copper piece with a solvent-based polish instead of wax.
- Be careful when cleaning patina or aged copper.
If the article helped at least a little, please like:
...or subscribe to the news:
Details
Corrosive qualities
Copper is a metal with high ductility properties that is reddish-golden in color and slightly pinkish after the oxide film is removed. In terms of electrical conductivity, it will be second only to silver, and is also characterized by a huge degree of thermal conductivity. Due to its low resistivity, copper is used in electrical engineering - it is used to make copper plates, electric motor windings, and wire.
Please note that due to its excellent anti-corrosion properties, the metal will be included in alloys to improve their engineering characteristics (brass, bronze, etc.). In a galvanic environment, copper turns into a cathode and begins to enter into electrochemical processes, and also causes an accelerated process of rusting of other metals.
Copper is an inactive chemical element, and therefore it practically does not interact with water (sea or fresh) or air. If the air is dry, an oxide film with a thickness of up to 50 mn will form on the surface of the material. The copper product begins to darken, becoming greenish or brown, and this is called patina. In many cases, patina is perceived as a decorative type of coating. The corrosive intensity is low when in contact with dilute hydrochloric acid, but when reacting with many other acids, with halogens, and “regia vodka,” the metal will be oxidized to form copper carbonate.
Conditions for copper destruction
Despite their resistance to damage, even copper products can rust under certain conditions. Such phenomena are least expressed in water, humid air, soil, and even more so in an acidic environment. Corrosion can be significantly reduced by tinning - coating copper with a tin layer. A high-quality tinning process provides reliability and protection from defects, and also increases resistance to corrosion, making the material not susceptible to high temperatures, hail, rain and snow. The service life of tinned products will be more than a hundred years without loss of original qualities.
Exposure to water
Protecting copper from corrosion is very important. The rate at which copper rusts in water will greatly depend on the presence of an oxide-type film on its surface, as well as on the level of oxygen saturation of the water. The more oxygen in the water, the more intense the destruction of the material will occur. In general, copper can be considered resistant to the harmful effects of fresh and salt water, and only dissolved chloride ions and a low pH level negatively affect it. Strength, as well as resistance to rust, makes it possible to use materials for the manufacture of pipelines.
Please note that if the surface of a product that is coated with copper has a green or even brown oxide crust, destructive components will penetrate inside to a small extent. As a rule, an oxide layer forms after 2 months of the metal being in water. The green crust (that is, carbonate) will be considered much stronger; the black crust (sulfate) will be loose and not so strong.
In sea water, the degree of corrosion is almost the same as in ordinary water, that is, fresh water. Only when the movement of water accelerates will rusting become impactful and therefore more intense. Copper is a material that cannot be overgrown by marine microscopic organisms because its ions are harmful to algae and mollusks. This property of the metal is used in shipping, as well as in fisheries.
Effect of alkalis and acids
Copper will not deteriorate in alkalis, because the material itself is alkaline, but acids will have the most negative effects for it. The fastest and most significant corrosion will occur upon contact with sulfur and its acidic types of compounds, and nitric acid can completely destroy the structure of the material. In concentrated acid, copper begins to dissolve, and therefore additional protection is required when manufacturing equipment for the oil and gas industry.
For this purpose, inhibitors are used - chemical reaction retarders:
- Shielding - creates a film that prevents acids from reaching the copper surface.
- Oxidizing - help turn the top layer into an oxide, which will begin to react with acids without harming the metal itself.
- Cathode - increase the cathode overvoltage, which slows down the reaction.
Let's look at some more things about corrosion.
Corrosion from moist air and soil
The soil is home to a large number of microscopic organisms that are capable of producing hydrogen sulfide, since the environment here is acidic, and the rate of copper corrosion will increase. The greater the deviation of the pH value towards oxidation, the faster destructive processes will occur. If the soil is equipped with oxygen, then the metal begins to oxidize, but it will rust less. When copper products remain in the ground for a long time, they begin to turn green, become loose and can even crumble. Short-term exposure to soil causes the formation of a patina, from which the item can be cleaned. By the way, humid air can have a bad effect on the condition of the material only with prolonged contact, and to begin with, it also causes the formation of a patina (oxide layer). The exception will be steam, which is saturated with sulfides, chlorides, carbon dioxide - corrosion will develop more rapidly in it.
How to coat copper to prevent it from oxidizing? — Machine tools, welding, metalworking
How to clean copper? The relevance of this issue is explained by the fact that products made from this metal have been used by humanity for many centuries. For a long time, the value of this metal was so high that it was equal to gold.
The development of technology has led to the fact that it was possible to significantly reduce the cost of copper production. This made it possible to make not only jewelry from this metal, but also dishes and interior items.
The high popularity of this metal and alloys based on it is explained not only by its decorative effect, but also by its unique characteristics - high ductility, thermal conductivity, corrosion resistance, etc.
No one wants to use oxidized copper cookware.
Why copper products need to be cleaned regularly
Regular cleaning of copper utensils and other items made from this metal is necessary because during use they quickly darken or become covered with a green coating - an oxide film.
To prevent copper from oxidizing
Forum Rules. Help Remember? Your messages Advanced search. Forum Electronic cigarette For beginners Question - answer How to paint copper fur correctly? Page 1 of 2 1 2 Last To page: Showing 1 to 20 of Topic: How to paint copper fur correctly?
Search data for your request:
Schemes, reference books, datasheets: Discussions, articles, manuals:
Wait for the search to complete in all databases. Upon completion, a link will appear to access the found materials.
WATCH THE VIDEO ON THE TOPIC: Protection of copper devices
How to coat copper products and is it worth it?
Your IP address will be recorded. Log in No account? Create an account. Remember me. Google. Previous Share Next. As an introduction, I’ll show you what I do: I make jewelry using the copper galvanization technique and sell them: , like this, for example: Most often I make hearts in one or another variation, this is the latest one I’ve made - custom paired keychains. The one with the round porthole was intended to be feminine, and the one with the bent panel was intended to be masculine.
The hearts themselves are made of polymer clay, which was coated with copper by galvanization. The metal is patinated, polished to a shine and waxed so that the copper does not oxidize and the polish remains as bright. The jewelry that is now in stock and on sale is the “Winged Heart” necklace. 2 This is the box the hostess will receive it in: polymer clay coated with copper by galvanization.
The metal is blackened, polished and coated with a protective varnish to prevent further oxidation. The back side is decorated with natural suede, so that even if the protective layer of varnish is damaged, the copper does not leave marks on the body. Wingspan 11.5 cm. The chain can be made to any length. It is better to store it separately, not in a jewelry box, so that the necklace does not get scratched on other metal objects, which can damage the protective layer of varnish, without which the copper will oxidize.
And when I get a little tired of hearts, I make this mechanical creature: The size from the beak to the tip of the tail is 7 cm. Something completely strange - a year and a half ago I made a steampunk Hammer of Thor for my man. He loves Scandinavian mythology and steampunk, so together we thought about how to bring these two directions together.
It turned out like this: design, crafts, retail store. Post a new comment Error. We will log you in after post We will log you in after post We will log you in after post We will log you in after post We will log you in after post Anonymously.
Your reply will be screened Your IP address will be recorded. Post a new comment. Preview comment. Post a new comment 52 comments.
What products can be used to coat copper jewelry?
Welcome, Guest. Please login or register. Gray az Guest. The actual question is, how do our forum members protect the brass and bronze elements of the knife from oxidation? Engineer Active participant Messages: Location: Nikolaev. I agree with Engineer, at work - no way.
To preserve the reddish hue of a red copper product, it is necessary to coat it with a special varnish to avoid oxidation. On air.
How to prevent copper from oxidizing
Dear craftsmen who work with copper, I know that the finished copper product also has a patina, can be coated with some kind of varnish to add shine, and the contact part in the earrings so as not to cause allergies? I'll stand nearby and listen. I suffer from things made of copper. It’s unpleasant and not beautiful.
We protect copper products
To preserve the reddish hue of a red copper product, it is necessary to coat it with a special varnish to avoid oxidation.
When exposed to air, copper first turns brown and then forms a greenish oxide, which must be dissolved because this patina preserves the underlying copper.
Currently, due to the emergence of new sources of environmental pollution, soluble copper salts are increasingly appearing in the air. They fall along with precipitation and pollute the facades of buildings. To prevent this, there are various preservative compositions that are applied to a well-cleaned surface of the product.
To clean copper products, it is best to use a 10% alcohol solution of hydrochloric acid. It is applied to the surface of the copper, then it must be thoroughly polished with cloth until it is completely shiny, after which the product is washed with clean water.
Attention! Hydrochloric acid is very caustic, so protect your eyes and wear safety glasses and rubber gloves. Pour the solution only into an acid-resistant vessel - acid corrodes ordinary metal vessels. Any remaining unused solution must be disposed of in a special waste container. After the copper has dried, it can be coated with a protective layer. For this purpose, we recommend two-component acrylic varnish, which is used for painting cars. This varnish is colorless and works well on copper (though it is relatively expensive). Depending on the type, the varnish is diluted in a ratio of 2:1 or 3:1 plus 10-15% thinner.
Two-component acrylic varnishes dry quickly. If you use a “slow” hardener, the drying process can be extended. One-component acrylic varnishes are less durable. For products located in interior spaces, you can use a combined nitro varnish (for example, “Zapon-varnish”). The popular alkyd varnish (the so-called artificial resin varnish) is unsuitable for this purpose, since, when combined with copper, it produces a green salt. The strength of the varnish coating largely depends on the thickness of the protective layer. Therefore, it is necessary to apply at least three layers with an interval of one day.
1. Copper has the property of changing color. If green patina spots appear, the product must be cleaned and coated with a protective varnish. Only in this case is it possible to preserve the natural color of copper.
2. Clean the surface of the copper product with a 10% alcohol solution of hydrochloric acid. Work only with rubber gloves.
3. Polish the surface to a metallic shine with an artificial fiber cloth. Rinse with water and let dry.
4. For preservation, coat the surface of the copper product with two-component colorless acrylic varnish.
Tools:
Sponge, polishing cloth, brush, roller or spray gun, safety glasses and gloves.
Protection of metals from corrosion and oxidation
Thin metal or organic surface coatings of metal products that improve their appearance, protect against corrosion, increase wear resistance, improve electrical contact, facilitate soldering, change reflective or absorption properties in the infrared and visible ranges of the spectrum, and also increase the size of the product. Silver, gold, nickel and chromium are applied to the surface of steel or other metals both to improve appearance and to protect against corrosion. Cadmium and zinc are used to protect against galvanic corrosion; these metals protect the steel through their own corrosion, and the degree of protection is almost proportional to the thickness or mass of the coating. Other metals used as coatings for steel, such as copper, nickel, chromium, tin, cobalt, silver, gold and lead, act as protective films; The degree of protection is proportional to the thickness only as long as the thickness ensures the impermeability of the coating. Thick chrome coatings are used primarily to increase wear resistance; cadmium and silver are used when it is necessary to ensure good electrical contact; tin, copper, cadmium and nickel are good soldering coatings; rhodium, silver and gold are used to increase the reflectivity of surfaces; black oxidation (bluing) is used to increase the absorption capacity and intrinsic radiation of the surface; Coatings of nickel, chromium and iron allow you to increase the size of parts.
The Saturn company wholesales and retails welding electrodes, semi-automatic welding machines, rectifiers and inverters. Dealer prices, discounts. Delivery.
To apply coatings to the surface of metal products, the following methods are usually used: application of organic coatings (paints, varnishes, enamels), oxidation, chemical treatment, diffusion metallization, immersion in melt, metal, sputtering and electrolytic deposition. Oil paints are used primarily for exterior decoration or to protect the surface of large metal structures; they dry so slowly that they are not suitable for coating most metal products. These disadvantages are absent from nitro varnishes, which were previously widely used to coat metal surfaces, such as cars, due to the fact that they dry quickly, form a durable film, have high adhesion and low cost, but are now being replaced by synthetic enamels. There are several types of commonly used oxidation processes. Aluminum is used as an anode material in a solution of sulfuric or chromic acid. The resulting oxide provides good protection of aluminum from corrosion and also serves as a good basis for applying organic coatings. In some cases, the oxide film can be dyed to achieve the desired color. Oxide films on the surface of steel are obtained by heat treatment, exposure to molten oxidizing agents (nitrates) and, most often, immersion in alkaline solutions heated to a temperature of 140-155 ° C. Copper and copper alloys are processed in alkaline solutions to obtain a film of black copper oxide. Red oxide forms on copper when it is immersed in an oxidizing melt. Silver, copper and brass are “oxidized” using sulfide solutions to produce colored and black coatings; these coatings are sulfides rather than oxides. Coatings obtained by chemical polishing are used to protect against corrosion and as a basis for applying organic coatings. For steel and zinc, a phosphating process is used using solutions containing metal phosphates and depolarizers; zinc and cadmium are processed in chromate solutions to produce chromium-containing coatings that are highly resistant to corrosion caused by salt aerosols; magnesium is also treated with chromate solutions to reduce corrosion and prepare it for painting; Buffer solutions of molybdates give a black coating on zinc. Some metals can be applied to the surface of products made from other metals by simple chemical displacement from a solution. Copper from a solution of copper sulfate in sulfuric acid can be deposited on steel; Even better results are obtained by adding an inhibitor to prevent the attack of sulfuric acid on the steel. Mercury can be replaced by copper and brass from cyanide solutions and form smooth, highly adhesive mercury coatings that are used to prepare brass for silver plating. Tin and zinc coatings are applied by dipping items into molten metal. Hot tin coatings are applied to steel tin (in the can industry), cast iron, ductile iron, copper and copper alloys, primarily in food contact and electrical applications. Proper preparation of metals for the electroplating process, similar to the application of organic coatings, requires the removal of all traces of lubricant, grease, particulate matter, oxide film and scale for proper coating application.
Copper-zinc alloy coatings can be prepared from cyanide solutions; they produce connections that resemble sheet brass. Brass coatings on steel and other products are often used for decorative purposes.
Real bronzes, i.e. copper-tin alloys can be prepared from cyanide solutions. The tin content in bronze coatings ranges from 5 to 10%.
admium coatings protect steel from electrochemical corrosion and are attractive in that they do not form white corrosion products, as is the case with zinc coatings. Cadmium coated parts are easy to solder and are therefore widely used in the electronics industry. Cadmium is toxic and should not be used in households or on products that will come into contact with food. There are two types of such coatings: thin (decorative) and thick (technological). The decorative coating is usually a shiny chrome layer with a thickness of 0.0005 to 0.0025 mm. A decorative coating with a thickness of 0.00075 to 0.0015 mm is applied to protect the nickel substrate from corrosion. A thick coating is, in principle, no different from a decorative one, but its greater thickness (from 0.025 to 0.375 mm) increases the wear resistance of the product. Copper coatings, which provide corrosion protection, are obtained from several types of solutions. For example, a solution of copper sulfate and sulfuric acid is used for electropolishing or producing thick coatings. Cyanide solutions are also used for coating steel, zinc, lead and other metals. Other metals precipitated from cyanide solutions are gold, silver and zinc. Iron is precipitated from solutions of ferric chloride, lead from solutions of fluoroborate and fluorosilicate, and tin from alkaline solutions of stannates and fluoroborate. Platings of noble metals such as rhodium, platinum and palladium can also be obtained by electroplating. Saturn LLC supplies wholesale and retail welding electrodes, semi-automatic welding machines, rectifiers and inverters. Dealer prices, discounts. Delivery. Call
Corrosion of aluminum, copper and other non-ferrous metals – we deal with corrosion + video
1 What is corrosion of metals and alloys?
In general, this process manifests itself as the destruction of a material as a result of its interaction with the external environment. Moreover, both metals and non-metals (ceramics, wood, polymers, etc.) are susceptible to it. We can also include the aging of rubber and the destruction of plastic here. What about metal alloys, in this case the most obvious example of corrosion is the well-known rust.
The main reason for this phenomenon is the insufficient thermodynamic stability of a particular material to any substances that we can detect in the contacting medium. For example, rubber coatings deteriorate due to interaction with oxygen, polymers are destroyed after numerous contacts with precipitation, and most metals and their alloys are detrimentally affected by excessive humidity. In addition, the ambient temperature also significantly influences the speed of the process; basically, the higher this parameter, the faster the destruction occurs.
2 Corrosion of copper and other non-ferrous metals - signs and features
In general, corrosion of aluminum and many of its alloys is quite rare, and all thanks to the characteristics of this metal - it is capable of passivation in various aggressive environments. In other words, it goes into a passive state, for example, when interacting with air, an oxide film is formed on its surface, which performs protective functions. Moreover, depending on the conditions, the thickness of the passive layer can be different.
The film is also resistant to moisture, but in an acidic environment there is no clear answer, it all depends on the type of acid. Thus, aluminum products are practically not afraid of either nitric or acetic acid (at normal temperatures), but oxalic, sulfuric, formic and salt have a detrimental effect on the metal. But this material is especially afraid of an alkaline environment, since when exposed to this substance, the aluminum oxide film is destroyed.
Now let's look at the cases in which corrosion of copper and alloys containing it occurs. This metal is destroyed when interacting with sulfur and its various compounds. She is also afraid of oxidizing and some aerated non-oxidizing acids, salts and heavy metals. What about the aquatic environment, in this case it all depends on how saturated it is with oxygen; the higher its content, the sooner destruction occurs.
Signs of corrosion of brass are expressed mainly in cracking (the intensity increases in a humid environment) and dezincification of this alloy, the latter being facilitated by solutions that contain chlorine ions. These processes also occur when the material interacts with ammonia, solutions of various oxidizing acids and salts. In addition, mercury, nitrogen oxides, ferric iron and copper are harmful to brass. Another cause of cracking can be tensile stress.
3 Protection of alloys and ways to stop corrosion
So, having learned a little about the peculiarities of the destruction of non-ferrous metals, it is worth paying attention to the question of how to stop unwanted corrosion of aluminum, its alloys and other materials described above. Of course, the best option would be to warn her, but for this you need to know some nuances.
For example, ultra-pure aluminum has maximum corrosion resistance; the most suitable environment should be selected for working with it and its alloys. In addition, protection can be carried out in such ways as creating a paint coating on the surface of the product, metallization, grinding or shot blasting, as a result of which residual compressive stresses arise.
If the metal is already damaged, then you need to thoroughly clean the damaged areas and treat them with special anti-corrosion solutions, which can be purchased quite easily on almost any construction market.
What about products made of copper and its alloys, in this case the control measures are almost the same as in the case of aluminum. Operating conditions, namely the pH of the environment, are less significant here; destruction will still be noticeable. Indeed, whether copper corrosion occurred in a highly acidic environment or in some other way, in any case the element needs thorough cleaning. Then protection is applied, which can be paint, varnish, oil or another metal such as tin and aluminum. The method where the surface is coated with a thin layer of molten tin is called tinning.
In order to prevent corrosion of brass as a result of dezincification, a little arsenic is added to its composition, this process is called alloying. Acid oxides can neutralize the effect of ammonia, but you also cannot overdo it with them. In addition, if we are talking about the manufacture of brass pipes and other products, then operations such as mandrelless drawing, as well as assembly with tension, should be abandoned in order to avoid the occurrence of tensile stresses. This provides a brief guide to corrosion protection for aluminum, brass, copper and their alloys. Of course, there are an incredible number of features, but it’s better to talk about this in separate articles.
Corrosion of metals/alloys – what is it?
Rusting refers to the process of destruction of metal under the influence of negative factors in the environment. To one degree or another, all metals and alloys will rust, and as a result of this, rust and places of deterioration in integrity (namely, holes) appear on them. Over time, all non-metals can begin to deteriorate - an example is the aging of rubber or even plastic from exposure to oxygen, constant contact with water, and also due to temperature changes.
The main root cause of rusting can be considered the thermodynamic instability of the metal to the influence of physical factors or even substances of chemical origin that are present in the contact medium. Compared to iron, copper will oxidize much less, but with increasing temperature this process will greatly accelerate. If regularly exposed to an environment at temperatures above +105 degrees, any metal will rust several times faster.
Protection of soldered surfaces from oxidation
On parts that have been cleaned of contaminants, in an atmosphere of even dry air, the formation of an oxide film is again observed, and its appearance is determined in seconds. Therefore, in order to avoid its occurrence, in some cases, coatings with high protective properties are applied to the soldered surfaces, for example, nickel, copper, silver, manganese, zinc, etc., in various ways, including galvanic, ion-plasma, electric arc, etc. The most Of these, galvanic is widely used in industry.
Source