Various anti-corrosion protection technologies are used for metal sheets and parts. Cathodic protection against corrosion has become widespread. This method has a number of characteristic features, and most often cathodic protection is used for large objects. These could be pipes, cars, metal pile structures, sea vessels. How exactly are pipelines protected from corrosion at the physical and chemical level?
Basic cathodic protection technologies
Cathodic protection is a special method of electrochemical protection of metal objects from rust and corrosion. The main principle is that a negative electrical potential is applied to the metal object being protected. This minimizes metal contact with external ions and substances with an electrical charge. The technology was developed approximately 200 years ago by British scientist Humphry Davy. To confirm his theory, he compiled several reports that were submitted to the government. Based on these reports, the world's first cathodic protection of a large industrial ship was carried out.
Anti-corrosion protection applies to various objects - pipelines, cars, roads, airplanes and so on. Please note that the type of metal does not matter - it can be iron, copper, silver, gold, aluminum, titanium and any other metal, as well as various alloys (with or without alloying additives). Corrosion protection of a car, individual pipe fragments, various decorative items of complex shape, and so on can be equally successful.
1 way
Connecting the part to an external source of electric current (usually compact substations perform this role). When the technology is used, the metal object acts as a cathode, and the electrical substation acts as an anode. Due to this, a shift in the electrical potential occurs, which makes it possible to protect the metal object from electrically active particles. The main areas of application of this technology are the protection of pipelines, welded structures, various platforms, road surface elements, and so on. This technology is quite simple and universal, so it is very popular in the world. Its main disadvantage is the need to connect the protective circuit to an external current source, which can be inconvenient in the case of objects that are located far from human civilization (this problem is partly solved through the use of autonomous energy sources).
Method 2
Galvanic polarization method (galvanic anode technology). This technique is also quite simple and intuitive: a metal object is attached to another that has a negative charge (most often this element is made of light metals - aluminum, zinc, magnesium). Galvanic polarization technology is usually used in cases where there is a protective layer on the surface of the object. This technology is popular in America, where there are a large number of sparsely populated areas and where there is a shortage of external energy sources. Experts argue that galvanic polarization could become very popular in Russia due to the peculiarities of our geography if a protective coating were applied to domestic pipelines (in such a scenario, the use of the first technology would be very difficult, forcing people to look for an alternative).
Tread protection (use of protector)
A type of cathodic protection is sacrificial. When using sacrificial protection, a metal with a more electronegative potential is connected to the protected object. In this case, it is not the structure that is destroyed, but the tread. Over time, the protector corrodes and must be replaced with a new one.
Tread protection is effective in cases where there is a small transition resistance between the protector and the environment.
Each protector has its own radius of protective action, which is determined by the maximum possible distance to which the protector can be removed without losing the protective effect. Protective protection is most often used when it is impossible or difficult and expensive to supply current to the structure.
Protectors are used to protect structures in neutral environments (sea or river water, air, soil, etc.).
The following metals are used to make protectors: magnesium, zinc, iron, aluminum. Pure metals do not fully perform their protective functions, so they are additionally alloyed during the manufacture of protectors.
Iron protectors are made from carbon steel or pure iron.
Zinc protectors
Zinc protectors contain about 0.001 - 0.005% lead, copper and iron, 0.1 - 0.5% aluminum and 0.025 - 0.15% cadmium. Zinc projectors are used to protect products from sea corrosion (in salt water). If a zinc protector is used in slightly salted, fresh water or soils, it quickly becomes covered with a thick layer of oxides and hydroxides.
Magnesium protector
Alloys for the manufacture of magnesium protectors are alloyed with 2–5% zinc and 5–7% aluminum. The amount of copper, lead, iron, silicon, nickel in the alloy should not exceed tenths and hundredths of a percent.
Magnesium protector is used in slightly salted, fresh waters and soils. The protector is used in environments where zinc and aluminum protectors are ineffective. An important aspect is that magnesium protectors must be used in an environment with a pH of 9.5 - 10.5. This is explained by the high rate of dissolution of magnesium and the formation of sparingly soluble compounds on its surface.
Magnesium protector is dangerous because... is the cause of hydrogen embrittlement and corrosion cracking of structures.
Aluminum protectors
Aluminum protectors contain additives that prevent the formation of aluminum oxides. Up to 8% zinc, up to 5% magnesium and tenths to hundredths of silicon, cadmium, indium, and thallium are added to such protectors. Aluminum protectors are used in the coastal shelf and flowing sea water.
Cathode polarization technology
In this case, the so-called superimposed current is used. An external conductor (often) or a current source (rarely) is used to apply it to a metal object. Upon contact with an electrically active particle, the following happens: the particle, under the influence of electrical attraction forces, moves to a protective element with a negative charge, where “recycling” of these particles occurs.
The consequences of such “disposal” are obvious - the protective element itself becomes corroded over time and becomes unusable. Therefore, this technology is often called the sacrificial electrode method (instead of our part, the “sacrificial electrode” rusts).
In addition to current and voltage, when working with cathodic polarization, one more important parameter must be taken into account - the ohmic voltage. In a technical sense, this parameter reflects the fact that as an electrical charge flows over time, the current voltage in the circuit drops. The drop itself occurs due to the fact that the cathode current flows along a circuit with a lower charge. If the circuit is assembled correctly, this indicator is quite small - thanks to this, the same current of the same power will always be maintained in the circuit.
Stress Corrosion Cracking
If a metal surface is simultaneously exposed to external negative factors and high voltage from power lines, which creates tensile forces, then rust formation occurs. According to the research carried out, the new hydrogen-corrosion theory gained its place.
Small cracks are formed when the pipe is saturated with hydrogen, which then ensures an increase in pressure from the inside to levels higher than the required equivalent of the bond of atoms and crystals.
After the crack opens, the rusting process of the metal accelerates, which is provided by the ground electrolyte. As a result, under the influence of mechanical influences, the metal undergoes slow destruction.
Technology for creating protection stations
Another technology for creating cathodic protection is connecting the element to external current sources. In most cases, for these purposes, special cathodic protection stations (CPS) are built, which consist of several elements - the main current source, anode grounding, various cables and wires connecting individual structural elements and auxiliary points with mechanical or computer control, which allow you to control the parameters .
Most often, this technology is used for objects located near power lines - these can be pipelines, various factory buildings, and so on. VCS can operate in multi-threaded mode - in this case they will serve several protection systems at once. On pipes, a practice has become widespread in which several separate blocks are placed on the pipes to distribute the current more efficiently. The thing is that in the case of long pipelines, in the places where the pipes are connected to current sources, special points with an increased level of electric field voltage are formed - because of this, damage to the pipes can occur. The use of such blocks allows electricity to be distributed evenly throughout the entire protective circuit.
Automation
Checkpoints can operate both manually and automatically:
- In the case of manual control, the change in voltage parameters is regulated by the operator. At the physical level, regulation is carried out by switching the operation of the transformer. The operation of the winding is regulated, which allows you to change the parameters of the electric current.
- In the case of automatic control, the change in voltage parameters is regulated by the device itself based on the parameters that were once set by the operator. At the physical level, control is carried out using special semiconductor thyristors. They turn on or off when the electric current parameters deviate from the specified parameters.
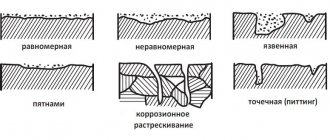
Main varieties
During corrosion in electrolytes, chemical energy is converted into electrical energy. In this regard, it is called electrochemical. It is customary to distinguish the following types of electrochemical corrosion.
Intercrystalline
Intergranular corrosion refers to a dangerous phenomenon in which the grain boundaries of nickel, aluminum and other metals are destroyed in a selective manner. As a result, the strength and plastic properties of the material are lost. The main danger of this type of corrosion is that it is not always visually noticeable.
Pitting
Pitting electrochemical corrosion is a point lesion of individual areas of the surface of copper and other metals. Depending on the nature of the lesion, closed, open, and superficial pitting are distinguished. The size of the affected areas can vary from 0.1 mm to 1.5 mm.
Slotted
Crevice electrochemical corrosion is commonly called the intensified process of destruction of metal structures in the locations of cracks, gaps and cracks. Crevice corrosion can occur in air, gas mixtures, and sea water. This type of destruction is typical for gas pipelines, the bottoms of sea vessels and many other objects.
Corrosion occurs in conditions of a small amount of oxidizer due to the difficult approach to the crack walls. This leads to the accumulation of corrosive products inside the gaps. The electrolyte contained in the internal space of the gap can change under the influence of hydrolysis of corrosion products.
In order to protect metals from crevice corrosion, it is common to use several methods:
- sealing gaps and cracks;
- electrochemical protection;
- inhibition process.
As preventive methods, you should use only those materials that are least susceptible to rust, and also initially correctly and rationally design gas pipelines and other important objects.
Competent prevention in many cases is a simpler process than subsequent cleaning of metal structures from ingrained rust.
Features of cathodic protection of pipes
Corrosion in pipelines usually occurs due to various defects and damage to the pipes - ruptures, cracking, cracks, and so on. Due to corrosion, the sealing of pipes is compromised, which can lead to complete or partial failure of the pipeline. This problem is especially acute for underground pipelines. When pipes are placed underground, areas with different electrical potentials are created. This is due to the heterogeneity of the soil and the presence of various debris of inorganic origin in the soil. If there is a serious potential difference, negatively charged ions in the ground begin to react with the metal. This leads to corrosion, which quickly destroys the pipeline.
Electric potential
Cathodic protection of pipelines against corrosion is carried out according to two standard schemes. Using cathodic polarization and by creating external stations. Pipeline protection should be aimed primarily at reducing the rate of destruction of the pipe material. This is done by reducing the electrical potential of the pipe in comparison with the electrical potential of the soil:
- The electrical potential of most modern pipes is approximately 0.8-0.9 volts.
- It has been experimentally shown that the main rocks of the soil have a potential of approximately 0.5-0.6 volts.
To equalize the electrical potentials, it is necessary to reduce the potential of the pipes by only 0.3-0.4 volts. This allows you to almost completely stop the appearance of rust. If the work is carried out correctly, the rate of natural rusting will be less than 1 mm per year.
Choosing a method
The technology for creating external protection stations is suitable for pipes. In this case, overhead power lines with voltages from 500 to 10,000 volts are used as power sources. The higher the voltage, the more pipes can be serviced. Sometimes there are no such lines in a particular area. In this case, it makes sense to install various generators.
External station technology has one major drawback. To create protection, labor-intensive and complex work will have to be carried out. This significantly increases the cost of creating a pipeline. When working with high voltage, excess electrical voltage may be created at the point of electricity supply - this can cause hydrogen cracking of pipes, so when carrying out installation work, electrical wiring must be done carefully.
Instead of the technology of protective stations, you can use the technique of using galvanic anodes to create a polarization effect. This technology is suitable for soils with low resistivity (up to 50 Ohms per 1 sq. m). If the soil resistivity is very high, then the technology of using galvanic anodes is practically useless due to its low efficiency.
Susceptibility to corrosion of main pipeline networks
Corrosion of pipelines of this type is the most well studied, and their protection from external factors is determined by standard requirements. Regulatory documents discuss methods of protection, and not the reasons for the formation of rust.
It is equally important to take into account that in this case only external corrosion is considered, to which the outer section of the pipeline is susceptible, since inert gases pass inside the pipeline. In this case, contact of the metal with the atmosphere is not so dangerous.
For protection against corrosion, according to GOST, several sections of the pipeline are considered: increased and high danger, as well as corrosion-hazardous.
Exposure to negative factors from the atmosphere for high-risk areas or types of corrosion:
- Stray currents arise from direct current sources.
- Exposure to microorganisms.
- The created stress provokes cracking of the metal.
- Waste storage.
- Salty soils.
- The temperature of the transported substance is above 300 °C.
- Carbon dioxide corrosion of an oil pipeline.
An installer for protecting underground pipelines from corrosion must know the design of the pipeline and the requirements of SNiP.
Features of cathodic protection of cars
Corrosion on cars often appears suddenly. The speed of its spread is very high, since the car has a large number of moving parts. During operation, various small cracks and dents may form in such elements. This significantly increases the risk of corrosion. Cathodic protection of a car against corrosion is usually carried out by redistributing the electrical potential.
Usually special electronic modules are used, which are compact in size and mounted inside the car. Installation of such blocks takes no more than 20 minutes.
Additional processing
It is also worth noting that the cathodic protection method is usually combined with other techniques:
- All main parts of the car are coated with special paints and mastics. They create a protective layer on the metal surface. This layer is electrically neutral. Therefore, upon contact with electrically active substances or ions, rusting does not occur.
- Some vehicle components may be coated with protective cathode plates, which also minimize the risk of rust. Plates are usually used to cover the moving parts that are most likely to crack and become damaged. This is the bottom of the car, rear wheel arches, headlights, interior door surfaces, and so on.
Causes of corrosion
Since the electrochemical method of protecting a car is aimed exclusively against corrosion, the reasons that cause it to damage the body should be considered. The main ones are water and road reagents used during the cold period. When combined with each other, they form a highly concentrated salt solution. In addition, dirt settled on the body retains moisture in the pores for a long time, and if it contains road reagents, it also attracts water molecules from the air.
The situation is aggravated if the car's paintwork has defects, even small ones. In this case, the spread of corrosion will occur very quickly, and even the remaining protective coatings in the form of primer and galvanization may not stop this process. Therefore, it is important not only to constantly clean the car from dirt, but also to monitor the condition of its paintwork. Temperature fluctuations as well as vibrations also play a role in the spread of corrosion.
You should also note the areas of the car that are most susceptible to corrosion. These include:
- parts located closest to the road surface, that is, sills, fenders and underbody;
- welds remaining after repairs, especially if they were carried out incorrectly. This is explained by high-temperature “weakening” of the metal;
- in addition, rust often affects various hidden, poorly ventilated cavities where moisture accumulates and does not dry out for a long time.