Introduction
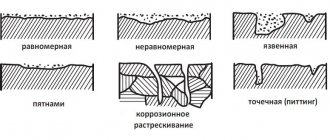
One of the most dangerous destructive phenomena for a steel pipeline is corrosion; in some areas it can reach 2-4 mm/year. In this regard, the construction of a main pipeline necessarily includes measures to protect the structure from corrosion, namely its insulation. Pipeline insulation can be passive (application of an insulating coating at the factory or on the route) and active (electrochemical protection). Moreover, passive insulation is effective from the beginning of the pipeline’s operation, and active insulation is activated after some time, depending on the aggressiveness of the soil.
This work discusses in detail one of the methods of electrochemical protection of a pipeline from soil corrosion - sacrificial protection.
How to provide protective protection
Coating pipes with special compounds is not only a task for the manufacturer; during the operation of the structure, ensuring protective properties must also be carried out. There are several ways to protect metal from aggressive environments:
- chemical treatment;
- coating the walls with special compounds;
- protection against stray currents;
- connecting the cathode or anode.
The method of tread protection of pipelines from corrosion is popular in organizations that install and operate pipeline transport.
About passive and active methods
Anti-corrosion protection is a whole range of measures carried out by enterprises. Passive protection methods involve performing the following work:
- At the installation stage, an air gap is left between the pipeline and the ground to prevent the ingress of groundwater, including those containing acidic and alkaline impurities.
- Coating with specialized compounds, the purpose of which extends from the negative effects of the soil.
- Treatment of metal with chemical compounds to form a thin film.
Active methods of protection involve the use of current and ion exchange based on chemical reactions, which ensures:
- Protection of underground pipelines from corrosion by creating an electrical drainage system to isolate pipeline transport from stray currents.
- Anode protection against destruction of metal surfaces.
- Cathodic protection to increase the resistance of metal substrates.
Only by taking into account all the methods that prevent the formation of rust on metal will the service life of structures be increased. Anti-corrosion protection of pipelines must be carried out comprehensively.
On video: protecting pipelines and cable lines from electrical corrosion.
About the advantages of using protectors
Pipe protection in this way is carried out with the addition of a component - an inhibitor. It is a material with a negative electrical charge. Under the influence of air masses it dissolves, but the structure remains intact and does not rust. Tread protection against corrosion is used to extend the service life of building structures, heating and water supply systems, as well as main and field pipeline transport.
The use of electrochemical protection makes it possible to eliminate the causes of many types of corrosion. Such anti-corrosion protection of pipelines is a good solution even for enterprises that do not have the financial capacity to provide full protection against an uncontrolled process.
To ensure a competent approach you should:
- Protectors made of aluminum are used in marine environments and coastal shelves.
- In environments with low electrical conductivity, use magnesium protectors. But, again, they are not suitable for treating the internal coating of tanks and oil settling tanks due to the fact that they have a fairly low fire and explosion hazard.
- Use protectors to protect against fresh water environments.
- Projectors made on the basis of zinc are completely safe and can be used in fire and explosion hazardous industries.
Protective anti-corrosion protection includes the following number of advantages:
- the lack of funds and production capacity of the enterprise will not be an obstacle to its implementation;
- the ability to protect small structures;
- if the pipes are covered with thermal insulation materials, then such protection is acceptable.
Materials used and purposes of application
Anti-corrosion protection is required for all metal substrates. This type of anti-rust treatment is widely used for treating tankers, since these vessels are most susceptible to exposure to water containing aggressive components. Even special painting cannot solve this problem.
The most rational choice for coating steel structures would be to use protectors with negative potential. In the manufacture of such devices, magnesium, zinc or aluminum is used. The large difference in potential between metal and steel surfaces helps to increase the spectrum of protective action, as a result, various types of corrosion are eliminated.
The protection system is carried out based on the specifics of the protectors themselves, as well as the environments in which they will be used.
Passive protection is required for steel coatings and metal products. The essence of the method is the use of galvanic anodes, which ensure that underground pipelines resist corrosion. When making calculations for this installation, the following indicators must be taken into account:
- current parameters;
- resistance against voltage surges;
- characteristics of the degree of protection used for 1 km of pipeline;
- indicator of the distance between protection elements.
Soil corrosion
Corrosion of metal pipelines is understood as their spontaneous destruction under the influence of various factors of a chemical or electrochemical nature, determined by the environment surrounding the pipeline.
Chemical corrosion is the spontaneous oxidation of a metal under the influence of a non-conductive environment. In this case, corrosion products are formed directly on the surface of the metal undergoing destruction.
Electrochemical corrosion is corrosion of metals in electrolytes, accompanied by the formation of electric current. In this case, the interaction of the metal with the environment is divided into anodic and cathodic processes occurring in different areas of the metal-electrolyte interface.
Soil corrosion belongs to electrochemical corrosion, but it has the following features:
1) connection of moisture with the environment:
— physical-mechanical connection (free water in the pores of the soil);
— physical-chemical bond (moisture adsorbed on the surface of soil or metal);
— chemical (hydrated) moisture included in the chemical compound Fe∙nH2;
2) heterogeneity of the structure and composition of the soil, both on the micro- and macroscales;
3) almost complete absence of mixing of the solid phase of the soil (slowing down the corrosion process over time);
4) unequal access of air oxygen to the metal surface.
The main reasons for the occurrence of corrosive elements on the pipeline
The conditions for corrosion to occur are:
— the presence of heterogeneity of soil areas with different potentials;
— presence of heterogeneous soil areas;
— the presence of means conducting electric current.
Reasons for the occurrence of corrosive elements on the pipeline:
1) micro-heterogeneity of the metal composition (presence of mechanical impurities in the metal of the pipes).
2) Presence of scale on the metal surface (microheterogeneity of the state of the metal surface).
3) The presence of longitudinal and transverse welds, which are the most dangerous areas in pipelines.
4) Various stressed states of the metal surface (stretched areas have a less negative potential).
5) Various pipeline depths.
6) Alternation of soils with different physical and chemical properties.
7) Temperature. With increasing temperature, the occurrence of anodic processes increases, i.e. the corrosion rate increases.
Methods for protecting pipelines
Corrosion of pipelines occurs during their operation. Rust formation occurs on pipes inside and outside. Deposits appear on the inside, and the reason for this is chemical reactions between the composition of the transported liquid and the metal. The condition of the surface is also influenced by high soil moisture.
If protection is not provided in a timely manner, a number of consequences may occur. What is important:
- It is recommended to carry out routine inspections at short intervals.
- Carry out repair work periodically, regardless of the presence of corrosion.
- suspension of the functioning of pipeline transport is inevitable, since it is necessary to carry out inspections and carry out scheduled preventative and other routine repairs.
Important! To ensure complete protection, it is necessary to take into account the installation method, contact with aggressive media, and the type of pipeline.
Conditions of use and principle of operation of tread protection of main pipelines against corrosion
Protective installations are intended:
— to protect long areas from soil corrosion, remote from power sources, where the use of cathodic protection by external current is inappropriate;
— in areas protected by SCZ, — in places of incomplete protection, to ensure the necessary protective potential;
— to protect cartridges (casings) from soil corrosion at crossings of railways and highways;
- in areas of stray currents - as earthen micro-drainages.
Protectors are also installed on insulating flanges to remove anodic zones, on electrical jumpers when jointly protecting underground structures to eliminate electrochemical interaction between them, to protect metal underground containers, etc.
The average tread life is 5-10 years.
Thus, the positive aspects of this ECP method:
- efficiency;
— simplicity of the device;
— ease of use;
— autonomy.
The negative aspects are a decrease in efficiency with a significant specific resistance of the soil surrounding the tread, and the use of scarce materials.
Fig.1. Schematic diagram of the tread installation:
1 – pipeline; 2 – drainage point; 3 – insulated connecting wire;
4 – protector; A – anode; K – cathode.
Tread protection of pipelines is based on the principle of operation of galvanic pairs. When protecting underground metal objects using protector installations, a protector (anode electrode) having a lower electrochemical potential than the potential of the pipe metal is connected to the pipeline. Conditions are created under which the pipeline acts as a cathode, and the electrode (protector) as an anode, as a result, the corrosive destruction of the pipeline is stopped due to the intensive destruction of the protector.
When installing protective protection, a metal protector is connected to the steel pipeline. As a result, a “pipe-protector” galvanic cell is formed, in which the pipeline is the cathode, the protector is the anode, and the soil is the electrolyte.
Thus, sacrificial protection has the same basics as cathodic protection. The difference is that the current necessary for protection is created by a large galvanic element, therefore sacrificial protection is otherwise called protection by galvanic anodes. In this case, the positive pole is located on the protected surface, and the negative pole is on the destructible anode, that is, in the reverse order to cathodic protection with imposed current from an external source.
Principle of cathodic protection
a) using galvanic sacrificial anodes.
b) using polarization from a direct current source.
1 - pipeline buried in the ground, 2 - galvanic sacrificial anode,
3 - direct current source, 4 - slightly soluble anode
The galvanic method is based on the fact that different metals in the electrolyte have different electrode potentials. If you form a galvanic couple from two metals and place them in an electrolyte, then the metal with a more negative potential will become the anode and will be destroyed, thereby protecting the metal with a less negative potential (Fig. 1.4a).
In practice, protectors made of magnesium, aluminum and zinc alloys are used as sacrificial galvanic anodes. The use of cathodic protection using protectors is effective only in low-resistivity soils (up to 50 Ohm.m). In high-resistivity soils, this method does not provide the necessary protection. Cathodic protection by external current sources is more complex and labor-intensive, but it depends little on the soil resistivity and has an unlimited energy resource (Fig. 1.4b). As a rule, converters of various designs powered from an alternating current network are used as direct current sources. The converters allow you to regulate the protective current over a wide range, ensuring pipeline protection in any conditions. 0.4 overhead lines are used as power sources for cathodic protection installations; 6; 10 kV, as well as autonomous sources: diesel generators, thermogenerators, gas generators and others. The operating principle and circuit of cathodic protection is shown in Fig. 1.4c
Operating principle and cathodic protection scheme
1-protected pipeline, 2-connecting wires, 3-DC source, 4-anode grounding, 5-points of damage to the insulating coating
The protective current applied to the pipeline from the converter and creating a “pipe-ground” potential difference is distributed unevenly along the length of the pipeline. Therefore, the maximum absolute value of this difference is located at the point of connection of the current source (drainage point). As you move away from this point, the potential difference “pipe-ground” decreases. Excessively increasing the potential difference negatively affects the adhesion of the coating and can cause hydrogenation of the pipe metal, which can cause hydrogen cracking. Reducing the potential difference does not provide corrosion protection and, within a certain range, may promote stress corrosion cracking. The main way to combat hydrogen cracking is to control the value of polarization potentials, especially at drainage points, and maintain it at a given level. Stress corrosion cracking is observed in a narrow range of pipeline potential, from minus 0.75 V to minus 0.83 V, and appears only under simultaneous exposure to high temperature and pressure, and also depends on the condition of the metal surface, the composition of the ground electrolyte and the condition of the insulation.
2.1. Metals and alloys used for the manufacture of protectors
Requirements for tread material:
— the protector material must have a more negative potential than the potential of the pipeline;
— dense oxide films should not form on the surface of the protector (the protector material should have low anodic polarizability);
— the tread material must have high efficiency, because self-corrosion of the tread occurs;
— the protector material must have a high specific current output, that is, g → max [A∙hour/kg];
— the amount of electricity per unit of weight (current output) should be maximum at minimum cost.
Aluminum, zinc and magnesium, as well as alloys based on them, are used as protector materials.
Table 1
Physicochemical properties of metals,
used as protectors
| Indicators | Magnesium | Zinc | aluminum |
| Relative molecular weight | 24,32 | 65,38 | 26,97 |
| Valence | 2 | 2 | 3 |
| Electrochemical equivalent, kg/(A∙year) | 3,97 | 10,7 | 2,94 |
| Current output, (A∙hour)/kg | 2200 | 820 | 2980 |
| Equilibrium electrode potential along a normal hydrogen electrode, V | -2,34 | -0,76 | -1,67 |
2.2. Fillers
Increasing the efficiency of the tread unit is achieved by immersing it in a special mixture of salts called an activator (also known as a filler). Direct installation of the protector into the ground is less effective than in the activator.
The purpose of the activator is as follows:
— reduction of self-corrosion;
— reduction of anodic polarizability;
— reducing the resistance to current spreading from the protector;
— eliminating the causes that contribute to the formation of dense layers of corrosion products on the tread surface.
When using an activator, a time-stable current in the “pipe-protector” circuit and a higher efficiency value (protector service life) are ensured.
The activator is prepared by mixing dry salts and clay with water to a viscous consistency according to recipes.
For one protector it is necessary to prepare 65-70 kg of activator.
table 2
Fillers and electrode potentials of protectors
from various materials
| Tread material | Aggregate composition | Electrode potential, V | |
| Ingredients | % | ||
| Magnesium | MgSO4 CaSO4 Clay | 25 25 50 | -1,7 |
| Aluminum | Ca(OH)2 NaCl Clay | 25 25 50 | -1,47 |
| zinc | Na2SO4 CaSO4 Clay | 25 25 50 | -1,2 |
Electrochemical protection of oil pipelines from corrosion
When metal comes into contact with soils related to electrolytic environments, a corrosion process occurs, accompanied by the formation of an electric current, and a certain electrode potential is established. The value of the electrode potential of the oil pipeline can be determined by the potential difference between two electrodes: the oil pipeline and the non-polarizing copper sulfate element. Thus, the potential value of an oil pipeline is the difference between its electrode potential and the potential of the reference electrode with respect to the ground. On the surface of the oil pipeline, electrode processes occur in a certain direction and changes in time are stationary in nature.
Stationary potential is usually called natural potential, implying the absence of stray and other induced currents on the oil pipeline.
The interaction of a corroding metal with an electrolyte is divided into two processes, anodic and cathodic, which take place simultaneously at different areas of the metal-electrolyte interface.
When protecting against corrosion, territorial separation of the anodic and cathodic processes is used. A current source with an additional grounding electrode is connected to the oil pipeline, with the help of which an external direct current is applied to the oil pipeline. In this case, the anodic process occurs on an additional grounding electrode.
Cathodic polarization of underground oil pipelines is carried out by applying an electric field from an external direct current source. The negative pole of the direct current source is connected to the protected structure, while the pipeline is the cathode in relation to the ground. An artificially created anode-grounding conductor - to the positive pole.
The schematic diagram of cathodic protection is shown in Fig. 1. With cathodic protection, the negative pole of the current source 2 is connected to the net pipeline 1, and the positive pole is connected to the artificially created anode-grounding conductor 3. When the current source is turned on, the current source from its pole through the anode grounding enters the ground and through damaged areas of insulation 6 to the pipe. Then, through the drainage point 4 along the connecting wire 5, the current returns again to the minus of the power source. At the same time, the process of cathodic polarization begins in the exposed sections of the oil pipeline.
Rice. 1. Schematic diagram of cathodic protection of an oil pipeline; 2 - external DC source; 3 - anodic grounding; 4 - drainage point; 5 - drainage cable; 6 — cathode terminal contact; 7 - cathode terminal; 8 - damage to oil pipeline insulation
Since the voltage of the external current applied between the grounding electrode and the oil pipeline significantly exceeds the potential difference between the electrodes of the corrosion macropairs of the oil pipeline, the stationary potential of the anodic grounding does not play a decisive role.
With the inclusion of electrochemical protection (j0a additional), the distribution of currents of corrosion macropairs is disrupted, the values of the potential difference between the pipe and the ground of the cathode sections (j0k) come closer to the potential difference of the anodic sections (j0a), and conditions for polarization are provided.
Rice. 2. Corrosion diagram for the case of complete polarization (a) and incomplete polarization (b)
Cathodic protection is regulated by maintaining the required protective potential. If, by applying an external current, the oil pipeline is polarized to the equilibrium potential (j0к=j0а) of metal dissolution (Fig. 2 a), then the anodic current stops and corrosion stops. A further increase in the protective current is impractical. At more positive potential values, the phenomenon of incomplete protection occurs (Fig. 2 b). It can occur during cathodic protection of an oil pipeline located in a zone of strong influence of stray currents or when using protectors that do not have a sufficiently negative electrode potential (zinc, protectors).
The criteria for protecting metal from corrosion are protective current density and protective potential.
Cathodic polarization of a bare metal structure to the protective potential requires significant currents. The most probable values of the current densities required for the polarization of steel in various environments to the minimum protective potential (-0.85 V) in relation to the copper-sulfate reference electrode are given in Table. 1.
Table 1
Current Density Required for Cathodic Protection of Bare Steel Surfaces in Various Environments
| Wednesday | Current density required for cathodic protection, mA/m2 |
| Sterile neutral soil | 4,3…16,1 |
| Well aerated neutral soil | 21,5…32,3 |
| Dry, well-aerated soil | 5,4…16,1 |
| Wet soil | 16,9…64,6 |
| Highly acidic | 53,8…161,4 |
| Soil that supports the activity of sulfate-reducing bacteria | 451,9 |
Typically, cathodic protection is used in conjunction with insulating coatings applied to the outer surface of the oil pipeline. Surface coating reduces the required current by several orders of magnitude. Thus, for cathodic protection of well-coated steel in soil, only 0.01 ... 0.2 mA/m2 is required.
The protective current density for insulated main oil pipelines cannot become a reliable protection criterion due to the unknown distribution of damaged oil pipeline insulation, which determines the actual contact area of the metal with the ground. Even for an uninsulated pipe (cartridge at an underground passage through railways and highways), the protective current density, determined by the geometric dimensions of the structure, is fictitious, because The portion of the cartridge surface that is covered with constantly present passive protective layers (scale, etc.) and does not participate in the depolarization process remains unknown. Therefore, protective current density as a protection criterion is used in some laboratory studies performed on metal samples.
As a criterion, GOST R 51164-98 “Main steel pipelines. General requirements for protection against corrosion”, the protective potential is accepted (Table 2).
table 2
Minimum protective potentials
| Conditions for laying and operating the pipeline | Minimum protective potential relative to a saturated copper sulfate reference electrode, V | |
| Polarizing | With ohmic component | |
| Soils with a maximum electrical resistance of at least 10 Ohm×m or a content of water-soluble salts of no more than 1 g per 1 kg of soil or at a temperature of the transported product of no more than 293 K (20°C) | -0,85 | -0,90 |
| Soils with a maximum electrical resistance of less than 10 Ohm×m or a content of water-soluble salts of more than 1 g per 1 kg of soil, or the dangerous influence of stray currents of industrial frequency (50 Hz) and direct currents, or with possible microbiological corrosion, or with a temperature of the transported product exceeding 293 K (20°C) | -0,95 | -1,05 |
Notes:
1. For pipelines whose temperature of the transported product is not more than 278K (5°C), the minimum polarization protective potential is equal to minus 0.80V relative to the saturated copper sulfate reference electrode.
2. Minimum protective potential with an ohmic component at the temperature of the transported product from 323K (50°C) to 343K (70°C) - minus 1.10V; from 343K (70°C) to 373K (100°C) - minus 1.15V.
3. For soils with high resistivity (more than 100 Ohm×m), the values of the minimum potential with an ohmic component must be determined experimentally or by calculation in accordance with ND.
A displacement of the pipe-ground potential difference in the negative direction relative to the minimum protective one is useless from the point of view of protection and causes an increase in current consumption. However, such a shift in the potential difference is necessary in places where cathodic protection stations (CPS) are connected to the oil pipeline in order to ensure a minimum protective potential difference in sections of the oil pipeline remote from the CPS. As soon as the potential difference between the pipe and the ground reaches values more negative than -1.10 V, the cathode process on the oil pipeline (cathode) will proceed with intense hydrogen release, which can disrupt the adhesion of the oil pipeline insulation. Therefore, for isolated oil pipelines, the maximum permissible potential difference is taken to be -1.10V (Table 3).
Table 3
Maximum protective potentials
| Conditions for laying and operating the pipeline | Maximum protective potential relative to a saturated copper-sulfate reference electrode, V | |
| Polarizing | With ohmic component | |
| When laying a pipeline with a temperature of the transported product above 333K (60°C) in soils with electrical resistivity less than 10 Ohm×m or when laying an underwater pipeline with a temperature of the transported product above 333K (60°C) | -1,10 | -1,50 |
| Under other pipeline laying conditions: | ||
| with bitumen insulation | -1,15 | -2,50 |
| with polymer insulation | -1,15 | -3,50 |
Notes:
1. For pipelines made of hardened steels with a tensile strength of 0.6 MPa (6 kg/cm2) and more, polarization potentials more negative than minus 1.10 V are not allowed.
2. In soils with high resistivity (more than 100 Ohm×m), more negative potentials with an ohmic component are allowed, established experimentally or by calculation in accordance with ND.
Observations have established that, under certain conditions, the insulating coating retains adhesion to the pipe even at more negative potential differences. This applies, first of all, to areas laid in well-aerated soils with a conscientiously executed coating. In sections of the oil pipeline where the insulation was done carelessly during construction with weak adhesion, it is observed to tear off the pipe under conditions of overprotection.
Studies have been carried out of the soil conditions in which pipelines are operated, in particular, the influence of soil moisture and pressure on the coating. The behavior of such new types of insulating materials as polymer materials and glass enamels under conditions of cathodic polarization has been studied. Experimental studies have established the fundamental possibility of using cathodic protection on underground steel pipelines with an increased protective potential compared to the norm in cases where the pipeline is not in constant contact with groundwater. Positive results were obtained by increasing the protective potential at the drainage point of cathode stations with bitumen insulation to -2.5 V, with polymer film and silicate enamels - to -3.5 V. This increase in the protective potential ensures an increase in the economic efficiency of cathodic protection of main pipelines due to reducing the number of cathode stations by 3 - 4 times.
For uninsulated steel pipes that do not have proximity or intersections with other metal structures, the shift of the potential difference in the negative direction is not limited.
The potential of an underground pipeline becomes more negative or more positive over time. It depends on the specific conditions. Two processes are continuously developing on the main oil pipeline:
1. Destruction of the insulating coating and the inclusion of more and more new, electrochemically active participants in the steel oil pipeline into the corrosion process. In this case, the stationary potential shifts to the negative side. An increase in humidity and natural compaction of the soil in the trench act in the same direction.
2. The formation of corrosion products and their deposits on the metal surface reduce its electrochemical activity and shift the stationary potential in a positive direction. This is also facilitated by drying out of the soil and drainage of groundwater from the oil pipeline route.
Depending on which of these processes is dominant under the conditions of a given oil pipeline, the nature of the potential shift will be determined.
When metal comes into contact with soils related to electrolytic environments, a corrosion process occurs, accompanied by the formation of an electric current, and a certain electrode potential is established. The value of the electrode potential of the oil pipeline can be determined by the potential difference between two electrodes: the oil pipeline and the non-polarizing copper sulfate element. Thus, the potential value of an oil pipeline is the difference between its electrode potential and the potential of the reference electrode with respect to the ground. On the surface of the oil pipeline, electrode processes occur in a certain direction and changes in time are stationary in nature.
Stationary potential is usually called natural potential, implying the absence of stray and other induced currents on the oil pipeline.
The interaction of a corroding metal with an electrolyte is divided into two processes, anodic and cathodic, which take place simultaneously at different areas of the metal-electrolyte interface.
When protecting against corrosion, territorial separation of the anodic and cathodic processes is used. A current source with an additional grounding electrode is connected to the oil pipeline, with the help of which an external direct current is applied to the oil pipeline. In this case, the anodic process occurs on an additional grounding electrode.
Cathodic polarization of underground oil pipelines is carried out by applying an electric field from an external direct current source. The negative pole of the direct current source is connected to the protected structure, while the pipeline is the cathode in relation to the ground. An artificially created anode-grounding conductor - to the positive pole.
The schematic diagram of cathodic protection is shown in Fig. 1. With cathodic protection, the negative pole of the current source 2 is connected to the net pipeline 1, and the positive pole is connected to the artificially created anode-grounding conductor 3. When the current source is turned on, the current source from its pole through the anode grounding enters the ground and through damaged areas of insulation 6 to the pipe. Then, through the drainage point 4 along the connecting wire 5, the current returns again to the minus of the power source. At the same time, the process of cathodic polarization begins in the exposed sections of the oil pipeline.
Rice. 1. Schematic diagram of cathodic protection of an oil pipeline; 2 - external DC source; 3 - anodic grounding; 4 - drainage point; 5 - drainage cable; 6 — cathode terminal contact; 7 - cathode terminal; 8 - damage to oil pipeline insulation
Since the voltage of the external current applied between the grounding electrode and the oil pipeline significantly exceeds the potential difference between the electrodes of the corrosion macropairs of the oil pipeline, the stationary potential of the anodic grounding does not play a decisive role.
With the inclusion of electrochemical protection (j0a additional), the distribution of currents of corrosion macropairs is disrupted, the values of the potential difference between the pipe and the ground of the cathode sections (j0k) come closer to the potential difference of the anodic sections (j0a), and conditions for polarization are provided.
Rice. 2. Corrosion diagram for the case of complete polarization (a) and incomplete polarization (b)
Cathodic protection is regulated by maintaining the required protective potential. If, by applying an external current, the oil pipeline is polarized to the equilibrium potential (j0к=j0а) of metal dissolution (Fig. 2 a), then the anodic current stops and corrosion stops. A further increase in the protective current is impractical. At more positive potential values, the phenomenon of incomplete protection occurs (Fig. 2 b). It can occur during cathodic protection of an oil pipeline located in a zone of strong influence of stray currents or when using protectors that do not have a sufficiently negative electrode potential (zinc, protectors).
The criteria for protecting metal from corrosion are protective current density and protective potential.
Cathodic polarization of a bare metal structure to the protective potential requires significant currents. The most probable values of the current densities required for the polarization of steel in various environments to the minimum protective potential (-0.85 V) in relation to the copper-sulfate reference electrode are given in Table. 1.
Table 1
Current Density Required for Cathodic Protection of Bare Steel Surfaces in Various Environments
| Wednesday | Current density required for cathodic protection, mA/m2 |
| Sterile neutral soil | 4,3…16,1 |
| Well aerated neutral soil | 21,5…32,3 |
| Dry, well-aerated soil | 5,4…16,1 |
| Wet soil | 16,9…64,6 |
| Highly acidic | 53,8…161,4 |
| Soil that supports the activity of sulfate-reducing bacteria | 451,9 |
Typically, cathodic protection is used in conjunction with insulating coatings applied to the outer surface of the oil pipeline. Surface coating reduces the required current by several orders of magnitude. Thus, for cathodic protection of well-coated steel in soil, only 0.01 ... 0.2 mA/m2 is required.
The protective current density for insulated main oil pipelines cannot become a reliable protection criterion due to the unknown distribution of damaged oil pipeline insulation, which determines the actual contact area of the metal with the ground. Even for an uninsulated pipe (cartridge at an underground passage through railways and highways), the protective current density, determined by the geometric dimensions of the structure, is fictitious, because The portion of the cartridge surface that is covered with constantly present passive protective layers (scale, etc.) and does not participate in the depolarization process remains unknown. Therefore, protective current density as a protection criterion is used in some laboratory studies performed on metal samples.
As a criterion, GOST R 51164-98 “Main steel pipelines. General requirements for protection against corrosion”, the protective potential is accepted (Table 2).
table 2
Minimum protective potentials
| Conditions for laying and operating the pipeline | Minimum protective potential relative to a saturated copper sulfate reference electrode, V | |
| Polarizing | With ohmic component | |
| Soils with a maximum electrical resistance of at least 10 Ohm×m or a content of water-soluble salts of no more than 1 g per 1 kg of soil or at a temperature of the transported product of no more than 293 K (20°C) | -0,85 | -0,90 |
| Soils with a maximum electrical resistance of less than 10 Ohm×m or a content of water-soluble salts of more than 1 g per 1 kg of soil, or the dangerous influence of stray currents of industrial frequency (50 Hz) and direct currents, or with possible microbiological corrosion, or with a temperature of the transported product exceeding 293 K (20°C) | -0,95 | -1,05 |
Notes:
1. For pipelines whose temperature of the transported product is not more than 278K (5°C), the minimum polarization protective potential is equal to minus 0.80V relative to the saturated copper sulfate reference electrode.
2. Minimum protective potential with an ohmic component at the temperature of the transported product from 323K (50°C) to 343K (70°C) - minus 1.10V; from 343K (70°C) to 373K (100°C) - minus 1.15V.
3. For soils with high resistivity (more than 100 Ohm×m), the values of the minimum potential with an ohmic component must be determined experimentally or by calculation in accordance with ND.
A displacement of the pipe-ground potential difference in the negative direction relative to the minimum protective one is useless from the point of view of protection and causes an increase in current consumption. However, such a shift in the potential difference is necessary in places where cathodic protection stations (CPS) are connected to the oil pipeline in order to ensure a minimum protective potential difference in sections of the oil pipeline remote from the CPS. As soon as the potential difference between the pipe and the ground reaches values more negative than -1.10 V, the cathode process on the oil pipeline (cathode) will proceed with intense hydrogen release, which can disrupt the adhesion of the oil pipeline insulation. Therefore, for isolated oil pipelines, the maximum permissible potential difference is taken to be -1.10V (Table 3).
Table 3
Maximum protective potentials
| Conditions for laying and operating the pipeline | Maximum protective potential relative to a saturated copper-sulfate reference electrode, V | |
| Polarizing | With ohmic component | |
| When laying a pipeline with a temperature of the transported product above 333K (60°C) in soils with electrical resistivity less than 10 Ohm×m or when laying an underwater pipeline with a temperature of the transported product above 333K (60°C) | -1,10 | -1,50 |
| Under other pipeline laying conditions: | ||
| with bitumen insulation | -1,15 | -2,50 |
| with polymer insulation | -1,15 | -3,50 |
Notes:
1. For pipelines made of hardened steels with a tensile strength of 0.6 MPa (6 kg/cm2) and more, polarization potentials more negative than minus 1.10 V are not allowed.
2. In soils with high resistivity (more than 100 Ohm×m), more negative potentials with an ohmic component are allowed, established experimentally or by calculation in accordance with ND.
Observations have established that, under certain conditions, the insulating coating retains adhesion to the pipe even at more negative potential differences. This applies, first of all, to areas laid in well-aerated soils with a conscientiously executed coating. In sections of the oil pipeline where the insulation was done carelessly during construction with weak adhesion, it is observed to tear off the pipe under conditions of overprotection.
Studies have been carried out of the soil conditions in which pipelines are operated, in particular, the influence of soil moisture and pressure on the coating. The behavior of such new types of insulating materials as polymer materials and glass enamels under conditions of cathodic polarization has been studied. Experimental studies have established the fundamental possibility of using cathodic protection on underground steel pipelines with an increased protective potential compared to the norm in cases where the pipeline is not in constant contact with groundwater. Positive results were obtained by increasing the protective potential at the drainage point of cathode stations with bitumen insulation to -2.5 V, with polymer film and silicate enamels - to -3.5 V. This increase in the protective potential ensures an increase in the economic efficiency of cathodic protection of main pipelines due to reducing the number of cathode stations by 3 - 4 times.
For uninsulated steel pipes that do not have proximity or intersections with other metal structures, the shift of the potential difference in the negative direction is not limited.
The potential of an underground pipeline becomes more negative or more positive over time. It depends on the specific conditions. Two processes are continuously developing on the main oil pipeline:
1. Destruction of the insulating coating and the inclusion of more and more new, electrochemically active participants in the steel oil pipeline into the corrosion process. In this case, the stationary potential shifts to the negative side. An increase in humidity and natural compaction of the soil in the trench act in the same direction.
2. The formation of corrosion products and their deposits on the metal surface reduce its electrochemical activity and shift the stationary potential in a positive direction. This is also facilitated by drying out of the soil and drainage of groundwater from the oil pipeline route.
Depending on which of these processes is dominant under the conditions of a given oil pipeline, the nature of the potential shift will be determined.