Electrochemical protection is an effective way to protect finished products from electrochemical corrosion. In some cases it is impossible to renew the paint coating or protective wrapping material, then it is advisable to use electrochemical protection. The coating of an underground pipeline or the bottom of a sea vessel is very labor-intensive and expensive to renew, sometimes simply impossible. Electrochemical protection reliably protects the product from corrosion, preventing the destruction of underground pipelines, ship bottoms, various tanks, etc.
Electrochemical protection is used in cases where the potential for free corrosion is in the area of intense dissolution of the base metal or repassivation. Those. when there is intense destruction of metal structures.
Main varieties
During corrosion in electrolytes, chemical energy is converted into electrical energy. In this regard, it is called electrochemical. It is customary to distinguish the following types of electrochemical corrosion.
Intercrystalline
Intergranular corrosion refers to a dangerous phenomenon in which the grain boundaries of nickel, aluminum and other metals are destroyed in a selective manner. As a result, the strength and plastic properties of the material are lost. The main danger of this type of corrosion is that it is not always visually noticeable.
Pitting
Pitting electrochemical corrosion is a point lesion of individual areas of the surface of copper and other metals. Depending on the nature of the lesion, closed, open, and superficial pitting are distinguished. The size of the affected areas can vary from 0.1 mm to 1.5 mm.
Slotted
Crevice electrochemical corrosion is commonly called the intensified process of destruction of metal structures in the locations of cracks, gaps and cracks. Crevice corrosion can occur in air, gas mixtures, and sea water. This type of destruction is typical for gas pipelines, the bottoms of sea vessels and many other objects.
Corrosion occurs in conditions of a small amount of oxidizer due to the difficult approach to the crack walls. This leads to the accumulation of corrosive products inside the gaps. The electrolyte contained in the internal space of the gap can change under the influence of hydrolysis of corrosion products.
In order to protect metals from crevice corrosion, it is common to use several methods:
- sealing gaps and cracks;
- electrochemical protection;
- inhibition process.
As preventive methods, you should use only those materials that are least susceptible to rust, and also initially correctly and rationally design gas pipelines and other important objects.
Competent prevention in many cases is a simpler process than subsequent cleaning of metal structures from ingrained rust.
Cathodic corrosion protection for a moving vehicle
Now let's figure out how to protect a moving car from corrosion with your own hands in this way. As in the method described above, the car acts as a cathode. We can use a grounded rubber “tail” or protective electrodes as an anode.
“Tail” is the simplest method of preventing rust. This is a strip of rubber with metallized elements attached. It is mounted on the back of the vehicle in such a way that it hangs down and creates a potential difference between the car and the wet road surface.
As humidity increases, the effectiveness of oxidation protection automatically increases. It receives splashes from under the wheels of the car, which is beneficial for the electrochemical process. An additional advantage of the “tail” is the removal of static voltage. For example, vehicles with flammable cargo even use such a means as metal chains that drag along the road - this removes the static charge, which can cause a spark and cause a fire.
Rubber grounding tail
The use of protective electrodes is suitable for both moving machines and stationary vehicles. To create an effective system, you need to install about 15-20 elements on the car. These are round or square plates ranging in size from 4 to 10 square centimeters. Aluminum, stainless steel, magnetite, graphite, and platinum are suitable for their manufacture. Aluminum and stainless steel deteriorate over time and will need to be replaced every 4 years.
Such elements have the following properties:
- operate within a radius of up to 0.35 m;
- placed only on painted areas of the car;
- attached using epoxy glue or putty;
- cleaning is required before installation;
- the outer side is not covered with any insulating materials;
- it is necessary to isolate the electrodes from the negatively charged car body
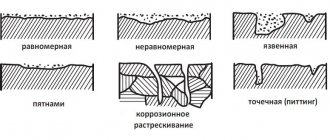
How different types of corrosion manifest themselves
An example of the corrosion process is the destruction of various devices, car components, as well as any structures made of metal and located:
- in atmospheric air;
- in waters - seas, rivers contained in the soil and under layers of soil;
- in technical environments, etc.
During the rusting process, the metal becomes a multi-electron galvanic cell. So, for example, if copper and iron come into contact in an electrolytic medium, copper is the cathode and iron is the anode. By donating electrons to copper, iron in the form of ions enters the solution. Hydrogen ions begin to move towards copper and are discharged there. Becoming more and more negative, the cathode soon becomes equal to the potential of the anode, as a result of which the corrosion process begins to slow down.
Different types of corrosion manifest themselves in different ways. Electrochemical corrosion manifests itself more intensely in cases where the cathode contains inclusions of metal with less activity compared to the corroding one - rust appears on them faster and is quite expressive.
Atmospheric corrosion occurs in humid air and normal temperatures. In this case, a film of moisture with dissolved oxygen forms on the surface of the metal. The process of metal destruction becomes more intense as air humidity and the content of gaseous oxides of carbon and sulfur increase, provided that:
- cracks;
- roughness;
- other factors that facilitate the condensation process.
Soil corrosion most affects a variety of underground structures, gas pipelines, cables and other structures. The destruction of copper and other metals occurs due to their close contact with soil moisture, which also contains dissolved oxygen. Destruction of pipelines can occur as early as six months after their construction if the soil in which they are installed is characterized by high acidity.
Under the influence of stray currents emanating from foreign objects, electrical corrosion occurs. Its main sources are electric railways, power lines, as well as special installations operating on direct electric current. To a greater extent, this type of corrosion provokes destruction:
- gas pipelines;
- all kinds of structures (bridges, hangars);
- electrical cables;
- oil pipelines.
The action of the current provokes the emergence of electron entry and exit areas - that is, cathodes and anodes. The most intense destructive process is in areas with anodes, so rust is more noticeable there.
Corrosion of individual components of gas and water pipelines can be caused by the fact that the installation process is mixed, that is, it occurs using different materials. The most common examples are pitting corrosion that occurs in copper elements, as well as corrosion of bimetals.
With a mixed installation of iron elements with copper and zinc alloys, the corrosion process is less critical than with copper casting, that is, with alloys of copper, zinc and tin. Pipeline corrosion can be prevented using special methods.
Corrosion prevention techniques.
Galvanization.
This type of protection is used by a considerable part of car manufacturers. In production, the surface of the machine is coated with fused zinc. With a sufficient layer of zinc, the technique is very effective. When the metal structure is damaged, the zinc surface is initially destroyed. This method of prevention protects your car from exposure to harmful chemicals.
Polymer protection.
The colorless film helps prevent rust from appearing on the surface of the machine. This type of protection is the most convenient and effective. The film is provided on an adhesive surface and is easily attached to the metal structure. Due to the lack of color, the film is very popular. Using this material, it is easy to protect the affected part of the car from unwanted influences.
Body lamination
body corrosion protection
You can protect your car body from abrasive wear and premature corrosion by using a special polymer film, which is an adhesive transparent material. In many cases, lamination is much more practical and convenient than other types of protection: Lamination is often used for partial protection of a car (doors, hood). The process of applying the film is quite simple and does not require much time. The material is resistant to temperature influences and is securely fixed to the surface. Thanks to this technique, you can maintain the original appearance of the car for a long time. This type of protection is often used before selling a car with low mileage. The film reliably protects the surface from scratches. Some dealers also use lamination to preserve the car during transportation.
Cathodic protection.
This technique involves the use of a special device. The device polarizes the metal, creating a protective layer. A negative charge accumulates on the surface of the body, preventing rust. The technique is effective for a certain period. If the polarization of the surface is destroyed, it is necessary to repeat the procedure. Therefore, cathodic protection works for some time. This method is used in hard-to-reach areas of the body. Some manufacturers use it in the area of the bottom of the car, thresholds and doors.
Barrier - protection.
Using plastic protection elements (for example, in the area of car wheels), you can prevent destructive particles from entering the car body. A barrier that blocks particles of sand and stones flying out from under the wheels. Thus, the varnish and paint surface retains its structure longer. Today, this protection is often installed by the manufacturer. There is also a special (plastic) protection for the bottom of the car. Some manufacturers use barriers in the area of pillars and other parts of the vehicle.
Primer.
One of the most popular types of rust prevention is applying a layer of primer. A small layer of primer is applied between the metal and the paint. This layer prevents moisture from getting on the metal. Protection is also effective for some time. It is not able to completely prevent rust, but it significantly slows down the process of surface destruction.
Protective paint.
underbody protection
A certain coating can not only decorate, but also protect the car. Paintwork is one of the most vulnerable. During the operation of the car, the paint constantly deteriorates
When choosing a paint, you need to pay attention to certain parameters.
Basic requirements for paintwork:
— High durability of the coating. - Resistance to abrasion. — Compatible with soil. — Safety for human health (low toxicity). — Resistance to thermal shock. Phosphate protection.
Rust resistance increases significantly when using a phosphate primer. This soil includes a number of chemical elements. The combination of elements forms a thin protective surface. In this case, exposure to external factors causes less damage to the metal coating and makes it more stable.
Car corrosion is difficult to eliminate. It is much easier to carry out prevention than to deal with emerging rust. The choice of protection method is a personal matter for each motorist. You shouldn't skimp on protecting your car body. Repairing and restoring the affected area is quite expensive. You can prevent damage to the body structure and costly repairs by using a protective layer. Thus, you will significantly increase the service life of the car body. Do not allow corrosion to occur, but prevent it from occurring. By protecting your car, you will remain confident in maintaining its appearance.
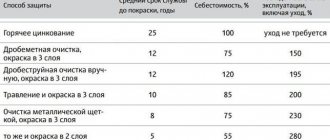
Methods of rust protection
Various methods are used to combat insidious rust. Let's look at those that are the most effective.
Method No. 1
One of the most popular methods is electrochemical protection of cast iron, steel, titanium, copper and other metals. What is it based on?
Electrochemical processing of metals is a special method aimed at changing the shape, size and roughness of the surface by anodic dissolution in an electrolyte under the influence of electric current.
To ensure reliable protection against rust, it is necessary to treat metal products with special means, which contain various components of organic and inorganic origin, even before using them. This method allows you to prevent rust from appearing for a certain time, but later you will have to renew the coating.
Scheme of cathodic protection of pipelines
Electrical protection is a process in which a metal structure is connected to an external source of direct electrical current. As a result of this, polarization of cathode-type electrodes is formed on its surface, and all anode regions begin to transform into cathode ones.
Electrochemical processing of metals can occur with the participation of an anode or cathode. In some cases, alternating processing of a metal product with both electrodes occurs.
Cathodic corrosion protection is necessary in situations where the metal to be protected does not have a predisposition to passivation. An external current source is connected to the metal product - a special cathodic protection station. This method is suitable for protecting gas pipelines, as well as water supply and heating pipelines. However, this method has certain disadvantages in the form of cracking and destruction of protective coatings - this occurs in cases of a significant shift in the potential of the object in the negative direction.
Method No. 2
Electrospark processing of metals can be carried out using various types of installations - non-contact, contact, and also anodic-mechanical.
Method No. 3
To reliably protect gas pipelines and other pipelines from rust, a method such as electric arc spraying is often used. The advantages of this method are obvious:
- significant thickness of the protective layer;
- high level of performance and reliability;
- use of relatively inexpensive equipment;
- simple technological process;
- possibility of using automated lines;
- low energy costs.
Among the disadvantages of this method are low efficiency when processing structures in corrosive environments, as well as insufficient adhesion strength to the steel base in some cases. In any other situations, such electrical protection is very effective.
Method No. 4
To protect a variety of metal structures - gas pipelines, bridge structures, all kinds of pipelines - effective anti-corrosion treatment is required.
This procedure is carried out in several stages:
- thorough removal of fatty deposits and oils using effective solvents;
- cleaning the treated surface from salts soluble in water is carried out using professional high-pressure apparatus;
- removal of existing structural errors, alignment of edges - this is necessary to prevent chipping of the applied paint coating;
- thorough cleaning of the surface using a sandblaster - this is done not only to remove rust, but also to give the desired degree of roughness;
- application of anti-corrosion material and an additional protective layer.
Correct pre-treatment of gas pipelines and all kinds of metal structures will provide them with reliable protection from electrochemical corrosion during operation.
DIY cathodic corrosion protection for cars in the garage
For a car that is stored motionless in a garage, organizing an electrochemical barrier with your own hands is very simple. As mentioned above, the machine itself acts as the cathode. The garage building itself can be designated as the anode if it is made of metal. Or it could be a ground loop if the garage is non-metallic, or the car is parked. A metal floor or exposed metal underneath will prevent rust from forming on the underside of the car.
The grounding loop is created in this way - we drive 4 metal pins into the ground around the machine. Their length must be at least 1 meter. We stretch a metal wire around these pins. The circuit is ready - unlike a metal building, it will only interact with the bottom of your car.
We connect the circuit or garage through a resistor - we connect it with the positive connector of the car battery.
We connect the circuit through a resistor to the battery
Electrochemical corrosion of metals and alloys
| Item: | Chemistry |
| Kind of work: | Course work |
| Language: | Russian |
| Date added: | 17.05.2019 |
- This type of work is not a scientific work, it is not a finished final qualifying work!
- This type of work is a finished result of processing, structuring and formatting collected information intended for use as a source of material for independent preparation of educational work.
If you have a hard time understanding this topic, write to me on WhatsApp, we’ll look into your topic, agree on a deadline, and I’ll help you!
At this link you can find many ready-made coursework in chemistry:
| Lots of ready-made coursework in chemistry |
Check out these similar threads, they might be useful to you:
| Chemical corrosion. Liquid and gas corrosion |
| Atmospheric corrosion of metals and methods of protection against atmospheric corrosion |
| Anodic and cathodic coatings. Cathodic and anodic protection |
| Main classes of inorganic compounds |
Introduction:
For galvanic corrosion to occur, the metal must be in contact with an electrolyte solution. In an aquatic environment, the mechanism of the electrochemical corrosion process is similar to that of a flashlight battery. Such an element consists of two electrodes: carbon - cathode and zinc - anode, separated by an electrolyte. Electrical energy is generated by chemical reactions that occur at each electrode. At the cathode there is chemical reduction, and at the anode there is oxidation, while zinc is converted into Zn2 + ions. In order for such processes to continue and the galvanic cell to work, it is necessary to close the circuit. However, the dissolution of zinc is also possible in an open circuit, in which case the role of the cathode is played by the smallest impurities in the zinc. Such elements are called microcorrosion. They play a major role in the process of electrochemical corrosion.
The surface of any metal consists of microelectrodes short-circuited through the metal itself. Microcorrosion elements begin to function when the metal comes into contact with the electrolyte, which ultimately leads to corrosive destruction of the metal. Thus, the corrosion process occurs and proceeds as a result of the work of many short-circuited galvanic microelements. The reasons for the formation of micropairs are very diverse: heterogeneity of the metal phase, liquid phase and physical conditions. Among the reasons for the heterogeneity of the metal phase, it should be noted the heterogeneity of the metal itself, the composition of the protective films on the metal and internal stresses in the metal.
Electrochemical dissolution of a metal is a complex process consisting of two main processes:
- The anodic process is the process of metal ions passing into solution, leaving a corresponding number of electrons on the metal surface:
- The cathodic process is the assimilation of excess electrons by atoms or molecules of the electrolyte (depolarizers), which are then reduced.
Thus, electrochemical corrosion on a non-uniform (heterogeneous) metal surface is similar to the operation of a short-circuited galvanic cell.
When two reversible electrodes of different potentials are sealed in an electrolyte, electrons flow from the more negative electrode (anode) to the less negative (or more positive) electrode (cathode). This flow of electrons equalizes the potential values of the closed electrodes. If in this case the electrode processes (anode on anode and cathode on cathode) do not continue, the electrode potentials will be equalized and complete polarization will occur. In fact, the anodic and cathodic electrode processes continue, preventing the onset of complete polarization due to the flow of electrons from the anode to the cathode, that is, they act depolarizing. Hence, in particular, the name of the ions and molecules of the solution that ensure the cathodic process occurs - depolarizers. However, due to the lag of the electrode processes from the flow of electrons in the galvanic cell, the potentials of the electrodes change (get closer) and the short-circuited system is ultimately completely polarized
Features of the electrochemical corrosion process:
- its division into two simultaneously occurring but largely independent electrode processes: anodic and cathodic;
- the dependence of the kinetics of these two electrochemical processes and, consequently, the corrosion rate in accordance with the laws of electrochemical kinetics on the potential value of the metal electrode: a shift in the metal potential in the positive direction (for example, as a result of polarization from an external current source) usually facilitates the anodic process and complicates the cathode; a potential shift in the negative direction, on the contrary, accelerates the cathodic process and slows down the anodic process;
- the possibility of localizing electrode processes in different areas of the surface of an aggressive metal, where their occurrence is facilitated;
- When electrode processes are localized, the material effect of corrosion (metal dissolution) is realized mainly in the anodic areas of the corroded metal surface.
Polarization, depolarization. Corrosion of metals with hydrogen depolarization. Corrosion of metals with oxygen depolarization
Some metals, such as magnesium, aluminum, are thermodynamically unstable from a thermodynamic point of view; in practice, under certain conditions, they corrode slowly and can be used as structural materials. In order to correctly assess the influence of various factors on the corrosion rate, it is necessary to have data on the state of equilibrium of the system and take into account the fact that when current passes through the electrode, its state of equilibrium is disturbed. The flow of electric current during operation of a corrosive microelement is determined by the initial potential difference between the cathode and anode; the potential of this electrode changes depending on the magnitude and direction of the external current.
The value of the corrosion current after closing the circuit quickly drops and after a certain time becomes constant. Since the value of the ohmic resistance in this case is small and constant, the decrease in the strength of the corrosion current can only be explained by mixing the initial values of the potentials, that is, a decrease in their difference. (figs). This change in potentials as a result of the flow of current is called polarization, and substances that reduce polarization are called depolarizers. A decrease in the polarizability of the electrodes is called depolarization.
Anodic polarization. There are three main cases, depending on the causes of anodic polarization.
Concentration polarization. This type of anodic polarization is due to the low diffusion rate of metal ions in the electrolyte solution, which leads to an increase in the concentration of ions in the anodic zone.
Metal ionization overvoltage
If during the anodic process the release of metal ions into solution does not keep pace with the removal of electrons, the metal potential shifts in a positive direction.
Anodic passivity, which consists in the formation of passive films on the metal surface. The anodic process is sharply inhibited due to the occurrence of the phenomenon of anodic passivity. This may explain the rather high corrosion resistance of aluminum and stainless steels in nitric acid solutions. Protective films on the anode can be formed as a result of the deposition of poorly soluble compounds of this metal on the metal surface.
Cathode polarization. This polarization is due to the overvoltage of the cathodic reaction, that is, the slowdown of the D + ne- <-> Dne process; concentration polarization, that is, insufficient rate of supply or removal of the initial or final reaction products at the cathode.
The following main types of cathodic depolarization are possible:
- depolarization by ions;
- cation discharge
- restoration of neutral molecules:
Corrosion of metals with hydrogen depolarization. The most common cases of corrosion in practice are processes occurring in solutions of non-oxidizing acids with the release of hydrogen - depolarization of hydrogen or in neutral salt solutions. Sometimes both of these processes can be performed in parallel.
Depolarization of hydrogen is thermodynamically possible in cases where the equilibrium potential of the metal is more negative than hydrogen under given conditions.
The process of discharging hydrogen ions at the cathode is presented in the form of a diagram consisting of several stages:
- Diffusion and migration of hydrated hydrogen ions to the cathode: H + H2O = (H3O) +
- Dehydration of hydrogen ions: (H3O) + -> H + + H2O
- Incorporation of a hydrogen ion into the bilayer
- Hydrogen ion discharge: H + + e- -> H
- Recombination of hydrogen atoms into a molecule: H + H = H2
- Formation and detachment of bubbles from hydrogen molecules from the cathode surface.
Corrosion of metals with oxygen depolarization. If there are no conditions for a corrosion process with depolarization of hydrogen or in the presence of oxygen in solution, then the process of reduction (ionization) of oxygen at the cathode, that is, the process of oxygen depolarization, plays the main role as a depolarizing reaction.
Thermodynamic possibility of the oxygen depolarization process: the equilibrium potential of the metal is more negative than the equilibrium potential of the oxygen electrode under given conditions.
The process of oxygen depolarization occurs in several stages, like any electrochemical heterogeneous reaction.
Corrosion protection methods
Depending on the nature of corrosion and the conditions under which it occurs, various protection methods are used. The choice of a particular method is determined by its effectiveness in a given particular case, as well as its economic feasibility. Any method of protection changes the course of the corrosion process, either reducing the rate or stopping it completely.
Protective coatings
Most metals and alloys tend to be unstable in the environment where they are used. Certain metals, such as aluminum, themselves protect against corrosion in some environments as a result of the formation of protective films on their surface when interacting with the environment. With the help of protective coatings, it is possible to isolate the metal from an aggressive environment by artificially applying a film to the surface of the product or by changing the chemical composition of the surface to make the metal resistant to an aggressive environment. The protective coating must be continuous, impermeable to aggressive environments, have high adhesion to the metal, be evenly distributed over the entire surface and give the product higher hardness, wear resistance and heat resistance. Protective coatings are also used to prevent mechanical wear of products and restore the dimensions of machine parts, as well as for protective and decorative finishing.
Protective coatings are divided into metallic and non-metallic, as well as coatings obtained by chemical and electrochemical treatment of metal surfaces.
Metal coatings and methods of their application
Metal coatings on products made of metallic or non-metallic (glass, ceramics, plastics, etc.) materials are applied to protect against corrosion, increase hardness, electrical conductivity and give them a beautiful appearance. Coatings are applied by electrochemical deposition (galvanic method), thermomechanical (cladding), sputtering (metallization) and immersion in molten metal (hot method).
Based on the nature of their protective effect against corrosion, metal coatings are divided into cathodic and anodic. Cathodic coatings include coatings in which the covering metal has a more positive electrode potential than the metal of the article being protected, such as tin- or copper-plated steel. If the integrity of the cathode coating is compromised, it ceases to protect the product from corrosion; in addition, the presence of such metal on the surface of the product increases its corrosion. Anodic coatings are those in which the coating metal has a greater negative electrode potential in a given environment than the protected one, such as when steel is coated with zinc.
The anodic coating protects the base metal when its integrity is destroyed, only being exposed to the destruction itself. Consequently, the requirements for tightness when applying an anodic coating are not very important, while the cathodic coating must be continuous and impermeable to aggressive environments.
Galvanic method. Electroplating is widely used in mechanical engineering because its application to products provides durable coatings at low cost and metal loss. The electroplating process involves separating and depositing a metal or alloy from aqueous solutions of its salts by passing a direct electric current through an electrolyte. The coated product in the electrolyzer serves as the cathode, and the anode serves as the deposited metal (soluble anodes), graphite, or metal insoluble in the electrolyte (insoluble anodes). Salts of these metals are used as an electrolyte, the metal of which is applied to the surface of the protected product.
Electroplating can be applied to a product with a thickness ranging from a micron to several millimeters. The coatings are very clean and evenly distributed over the entire protected surface. The electroplating method has a number of advantages over other methods and, in particular, is characterized by ease of control of the coating thickness, low metal consumption, does not require heating of the electrolyte, and ensures the deposition of both metals and alloys, but the coating obtained by this method is porous.
Galvanizing of cast iron and steel products is widely used in practice. This is explained by the fact that zinc in relation to steel in aqueous solutions serves as an anode (anode coating), therefore during electrochemical corrosion it dissolves and protects the product from destruction. Zinc is resistant to atmospheric corrosion. The thickness of the coatings varies and ranges from 3 to 50 microns.
Cadmium is applied almost exclusively by electroplating. It is used to protect parts of steel equipment from corrosion in sea water, humid air, and solutions of certain salts and alkalis. The thickness of the cadmium coating depends on the operating conditions and ranges from 9 to 15 microns for normal conditions and up to 45 microns for parts exposed to sea and hot water.
Tin is resistant to moist air and weak organic acids. In relation to iron, tin is a cathodic coating, so it protects steel products from corrosion mechanically in the absence of pores. The thickness of the coatings ranges from 1.5 to 20 microns.
Lead is a chemically resistant element. Resistant to sulfuric acid (up to 75%), solutions of its salts, sulfur-containing gases, weak hydrochloric acid. The thickness of the coating ranges from 30 to 200 microns.
Copper is not resistant to atmospheric corrosion, as it easily reacts with water vapor, carbon monoxide (IV) in the air, sulfur-containing gases and other media. Therefore, it is not used to protect steel from corrosion, but is widely used to obtain multi-layer protective and decorative coatings as an intermediate layer, for example, copper-nickel-chromium. In addition, copper is used to improve soldering and increase the electrical conductivity of products. The thickness of copper coatings is 5-30 microns or more.
Nickel is primarily used as a protective and decorative coating, but it can protect steel products from corrosion provided the resulting films are porous. Nickel coatings have high hardness and wear resistance. They are resistant to alkali solutions and organic acids, but are destroyed by mineral acids and solutions containing ammonia. The thickness of the coatings ranges from 3 to 40 microns.
Chrome plated products have high surface hardness, wear resistance, heat resistance and chemical resistance. They are stable in concentrated nitric acid, alkaline solutions, organic acids, hydrogen sulfide, and solutions of many salts. Chrome coatings are used for protective and decorative purposes, in the production of mirrors, reflectors, spotlights, to increase the wear resistance of products and to restore the dimensions of parts. The thickness of the coatings, depending on the purpose, is 3 - 250 microns or more.
Silver plating is mainly used to impart high electrical conductivity to parts, to coat products used in the food and art industries, as well as in many branches of mechanical engineering. Silver is used for steel, copper, brass and other materials.
Gold is used to protect valuable equipment and devices from atmospheric corrosion in jewelry and watchmaking. Used for copper and its alloys, nickel, steel - after copper plating - and other metals. Products with galvanic coating made of platinum, indium, germanium, gallium and other metals.
Hot method. The property of metals to have high chemical activity in the molten state formed the basis for the technology of applying a metal deposit to the product. The high reactivity of the molten metal in most cases results in the formation of an alloy with the product metal, which is a good binding base for the metal being coated. For hot metal coating, the workpiece is immersed for a few seconds in a bath of molten metal, which wets their surface. Hot coating uses a metal with a lower melting point than the metal being coated.
Ferrous metal parts are coated with lead, which are then widely used in the chemical industry. The lead coating is non-porous, resistant to many electrolytes and, importantly, to the effects of dilute solutions of sulfuric acid and its salts, and sulfur dioxide gases.
Zinc is used to protect steel and cast iron products from atmospheric corrosion, water and a number of neutral salt solutions. Zinc is applied to parts by immersing them for 6 - 20 seconds in molten zinc or zinc with the addition of aluminum or tin at 440 - 460 ºС. Tin is used to protect copper wires from sulfur, to produce tinplate, and to locally protect the metal surface during nitriding.
Aluminum is used for products made of iron, steel, and cast iron in order to increase resistance to atmospheric and gas corrosion. The high protective properties of aluminum coating are associated with the presence on the surface of a homogeneous dense film of aluminum oxide, formed as a result of the oxidation of aluminum under the influence of atmospheric oxygen.
Using the hot coating method, it is impossible to obtain deposits of uniform thickness, and also to protect products with narrow holes and threads from corrosion. The consumption of non-ferrous metals when using the hot method is high, since the resulting deposits are thick. In addition, this method allows only metal deposits to be applied to small objects.
Facing. This is the process of protecting a base metal or alloy from corrosion by another metal (alloy) that is resistant to an aggressive environment. Two metals can be joined together by casting, rolling and undeformed shell. The most widely used method is the joint rolling of two metals, one of which (chemically resistant) is protective. Metals or alloys with good weldability are used for cladding. These include carbon, acid-resistant steels, duralumin, and copper alloys. Stainless steel, aluminum, nickel, titanium, tantalum are used as facing materials. The thickness of the facing layer ranges from 3 to 60% of the thickness of the base metal.
The metal cladding method is the basis for obtaining a material called metal-plastic. It is produced by rolling or gluing a metal sheet and one or two polymer sheets. Metal-plastic is made from steel, aluminum and magnesium alloys, and thermoplastic polymers are used as a protective layer against corrosion: PVC, polyisobutylene, polyethylene, polypropylene, etc. The cost of metal-plastic is much cheaper and more durable than stainless steel, and it is superior in chemical properties resistance to aggressive environments... Metallization spray. This is the process of applying protective coatings to the surface of products by spraying molten metal or other refractory material with compressed air or inert gas. Metallization is carried out in metallizing equipment.
During metallization, the coating is formed due to jamming, the adhesion of molten particles of metal or other material to the pores and uneven surfaces of the part. Its adhesion force depends on the size of the particles, their flight speed and the amount of their deformation when they hit the surface. The coating has a scaly structure and high porosity, which is reduced by increasing the thickness of the coating, grinding, polishing and applying non-metallic coatings. For example, the porosity of a zinc coating decreases 10 times when its thickness increases from 0.2 to 0.4 mm.
The main disadvantages of metallization, despite its simplicity, are the high consumption of metal, the porosity of the resulting coating, low adhesion of the coating to the metal and the difficulty of adjusting the thickness of the coating layer.
Non-metallic coatings
Non-metallic coatings are divided into paints and varnishes, coatings with polymer materials, rubber, silicate enamels, lubricants, and pastes. They are used to protect metal products, equipment and structures from corrosion, insulate parts of electrical machines, protect products from rotting, moisture and give them a beautiful appearance.
Paint and varnish. The greatest use of non-metallic coatings is observed in paint and varnish coatings. The raw materials for producing paint and varnish coatings are film-forming substances, fillers, solvents, plasticizers, pigments, and catalysts. Film-forming substances are the basis of the coating.
To reduce the cost of the coating, increase corrosion resistance and heat resistance, fillers are introduced into its composition: talc, mica, kaolin, asbestos dust, spar, etc. Increasing hardness, heat resistance, mechanical strength, waterproofing, imparting a certain color by introducing pigments into the coating composition.
The coating process consists of several stages:
- preparing the metal surface for coating;
- applying a primer that improves the adhesion of the coating to the metal;
- applying an intermediate layer to level the surface;
- applying one or more layers of coating to the surface of the product;
- polishing the coating to obtain a lasting shine.
Drying oils, varnishes, paints, enamels, primers, putties are prepared from paints and varnishes. Varnishes are solutions of film-forming substances in highly volatile organic solvents. When pigments are added to drying oils, oil paints are obtained. Enamel paints are prepared by introducing pigments into varnishes. Primers are used to ensure strong adhesion of the coated product. Primers give the coating anti-corrosion properties. Elimination of marks, grooves, and defects is achieved using putty - a paste consisting of pigments, fillers, and film-forming substances.
According to their purpose, paint and varnish coatings are divided into those that are resistant to aggressive environments (that is, resistant to atmospheric corrosion, chemicals, oils, benzene), heat (heat-resistant) and mold; conductive, anti-fouling, luminous; decorative.
Polymer coating. To protect against the influence of the external environment, parts, products, and structures are coated with resins—polymers. The resin is applied in the form of a melt or suspension by brushing, dipping, or spraying. After heating and cooling, a polymer film several millimeters thick is formed on the surface of the part.
Gumming. Rubber and ebonite coatings of chemical devices, pipelines, tanks, containers for transporting and storing chemical products, etc. are called gumming. Depending on the conditions of the technological process, the nature of the environment, the type and method of coating. Soft rubbers containing 2.0 to 4.0% sulfur are used for gumming devices subject to impact, impact, sudden temperature changes or containing suspended matter, as well as for devices operating at constant temperature, not subject to mechanical stress, hard rubbers ( ebonites) containing from 40 to 50% sulfur. To obtain a dense protective layer, rubber and ebonite are often combined.
Electrochemical protection
The use of anti-corrosion materials, as well as the application of protective coatings, are not always suitable for combating corrosion. The painting of metal structures must be periodically renewed, which is associated with high costs. Sometimes this cannot be done, for example, on pipelines and cables laid in the ground, structures in sea water.
Using electrochemical protection, it is possible to prevent the destruction of pipelines, ship hulls, tanks and reaction apparatuses, etc. With electrochemical protection, a permanent strong anode (protector or direct current source) is connected externally to metal structures, which causes cathodic polarization of the electrodes of microgalvanic pairs on the surface of the protected metal, as a result of which the anodic sections of the metal structure are converted into cathodic ones. This means that it is not the metal of the structure that will collapse, but the attached anode. Electrochemical protection is divided into cathodic and anodic.
Cathodic protection
Cathodic protection is widely used both as an additional (to the insulating coating) and as an independent means for protecting metal structures of underground structures, gas pipelines, tanks, etc. from corrosion.
The essence of cathodic protection is that the negative pole of the direct current source or protector (cathode) is connected to the structure being protected, and the positive pole of the current source is connected to a metal or graphite plate (anode). The corrosive metal can be considered as a short-circuited multielectrode cell. The transition of metal ions from the anode sections into the solution and electrons from the anode sections into the cathode sections, the restoration of depolarizers at the cathode cause the appearance of an electric current and, as a consequence, metal corrosion. Therefore, by connecting the negative pole of the current source to the protected product, we turn it into a cathode, that is, we prevent destruction. With this connection, the DC source will be discharged and the metal plate, which is the anode, will be destroyed.
Protective protection is achieved by attaching a metal plate to a structure whose potential is more negative than the potential of the metal of the structure. Protective protection is used for structures operating in saline solutions and sea water. The use of protectors to protect equipment operating in tap or river water is disadvantageous due to their low electrical conductivity, since in this case large protectors must be installed. In highly aggressive environments, it is not advisable to use such protection due to the rapid destruction of the tread. When protecting steel structures, cast iron and protectors made of magnesium, zinc or an alloy containing 90% aluminum and 10% zinc are used.
Anodic protection
A number of metals, such as chromium, nickel, titanium, zirconium and iron alloys containing these metals, easily pass into a passive state, which is stably maintained in oxidizing environments. This property is of great practical importance in corrosion protection. The formation of films on metals leads to a negative shift in the potential of the active metal, which reduces the corrosion rate. Consequently, it is possible to artificially create a passive state of the metal surface due to anodic polarization from an external source of electrical energy current, which will reduce the corrosion rate by several orders of magnitude with minimal energy consumption, since the current is very small. This type of corrosion control is called anodic protection, which is recommended for use in very aggressive environments. Anodic protection can be performed in several ways, but most often by simply applying a constant EMF from an external current source. In addition, unlike cathodic protection, the positive pole of the current source is connected to the product being protected, and the cathodes are located near the surface of the product. The number of cathodes, their sizes and distance from the product must ensure uniform anodic polarization. Anodic protection is used to prevent damage to alloy steel products. The effectiveness of anodic protection can be observed if we take into account the corrosion of 10Х18Н9Т steel in 50% sulfuric acid at 50 ° C.
Nowadays, anodic protection is also used in industry to protect carbon steel products, thereby increasing the service life of equipment. The use of anodic protection reduces contamination of the aggressive environment with corrosion products. For example, the iron content of oleum in a steel device with anodic protection is only 0.004%, while in a device without anodic protection, the iron concentration in oleum increases sharply and becomes equal to 0.12%.
Corrosion inhibitors
It is also possible to reduce the rate of corrosion of metal equipment by changing the composition of the aggressive environment. This is achieved by removing corrosives (substances that increase corrosion) or by introducing compounds that inhibit and sometimes completely stop the corrosion process. Can be removed from aggressive substances that increase the rate of corrosion by boiling solutions, passing inert gases through them, chemically treating the environment, etc.
Oxygen, which is present in an aggressive environment, causes great harm to equipment, which sharply increases the rate of corrosion. Therefore, water or aqueous solutions of salts, where corrosion of metals and alloys occurs due to depolarization of oxygen, undergoes deoxygenation or deaeration. For example, a sample of steel in raw water begins to deteriorate after a few minutes, whereas after boiling the water does not react with the steel for a long time. This is because gases, particularly oxygen, have been removed from the water. If such water is isolated from contact with air, that is, the dissolution of oxygen in it is excluded, then the steel will not corrode for many months and even years. But this method of reducing the corrosion rate of apparatus and equipment is cumbersome and labor-intensive, so it is of limited use.
It is possible to reduce or completely eliminate corrosion of metal equipment by introducing compounds into an aggressive environment, which significantly reduces the corrosion process. This method of reducing the rate of corrosion is called inhibition, and the substances introduced into the medium are called corrosion inhibitors or inhibitors. Inhibition is used only in systems with a constant volume of aggressive solution, for example, when protecting tanks, tanks, pickling baths, etc. The concentration of the inhibitor introduced into the medium depends on the composition and properties of the medium, temperature, pH. decision, etc.
The mechanism of action of corrosion inhibitors in most cases is electrochemical in nature, that is, some substances introduced into the medium slow down the anodic process, while others slow down the cathodic process or both stages of the process at the same time.
Based on the nature of their protective effect, the inhibitors used are divided into cathodic, anodic and organic.
Substances with oxidizing properties (chromates, dichromates, nitrites, etc.) are classified as anodic retarders. They form passive, most often oxide films ≈0.01 μm thick, on the surface of a metal or alloy anode and reduce the rate of its dissolution.
Cathode retarders include substances that can slow down individual stages of the cathodic process. For example, in processes associated with oxygen depolarization, the corrosion rate decreases with decreasing oxygen concentration in the solution. Therefore, the introduction of oxygen scavengers into the solution (Na2SO3) reduces the corrosion rate. Corrosion can be reduced by using cathodic retarders to reduce the surface area of the cathode zones. These include ZnSO4, ZnCl2, etc. The reduction in corrosion with the introduction of these compounds is explained by the formation of the insoluble compound Zn (OH) 2 in an alkaline medium, which, deposited on the walls of the apparatus, isolates the cathode surface areas from contact with the solution.
Conclusion:
Anodic and cathodic retarders (usually inorganic compounds) reduce corrosion in neutral and alkaline environments, but do not have a protective effect in acidic environments.
Organic corrosion inhibitors include organic colloids, surfactants and other compounds. Organic inhibitors are adsorbed on the surface of the metal, and not on the entire surface, but only on its cathode, active sites that prevent the discharge of hydrogen ions and, as a result, the destruction of the metal. Organic moderators are not adsorbed by the oxidized surface or corrosion products on it, so they will decompose when exposed to an aggressive environment. This property of organic moderators is used in acid etching of metals, when rust and scale are dissolved without noticeable corrosion of the metal.
The protective effect of organic moderators depends on the nature of these substances, temperature and their concentration in an aggressive environment. As the temperature rises, their surface adsorption decreases and the protective effect drops sharply. The concentration of the inhibitor in the solution must be strictly determined.