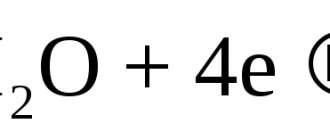Corrosion rate is a multifactorial parameter that depends on both external environmental conditions and the internal properties of the material. In the regulatory and technical documentation there are certain restrictions on the permissible values of metal destruction during the operation of equipment and building structures to ensure their trouble-free operation. In design, there is no universal method for determining corrosion rate. This is due to the complexity of taking into account all factors. The most reliable method is to study the operating history of the facility.
Criteria
You might be interested in:Isobaric, isochoric, isothermal and adiabatic processes for an ideal gas
Currently, several corrosion rate indicators are used in equipment design:
- According to the direct method of assessment: reduction in the mass of a metal part per unit surface - weight indicator (measured in grams per 1 m2 per 1 hour); depth of damage (or permeability of the corrosion process), mm/year; the amount of released gas phase of corrosion products; the length of time during which the first corrosion damage appears; the number of corrosion centers per unit surface area that appeared over a certain period of time.
- According to indirect assessment: current strength of electrochemical corrosion; electrical resistance; change in physical and mechanical characteristics.
You may be interested in: Such ordinary people, or the meaning of “why not”
The first indicator using the direct assessment method is the most common.
Corrosion of screw piles. How to extend the service life of a pile-screw foundation?
For foundations on screw piles, the greatest danger is posed by two subtypes of electrochemical corrosion - soil and atmospheric.
Soil corrosion is the destruction of underground metal structures under the influence of soil electrolyte. On the surface of metal products in contact with soil electrolyte, due to local inhomogeneities of the metal or electrolyte, a large number of corrosive elements appear.
However, we must not forget that soils and soils are extremely diverse, not only within large regions, but also within one small area. That is, in a relatively small area there can be soils with varying degrees of corrosive aggressiveness: highly corrosive (heavy clayey soils that retain moisture for a long time), moderately corrosive (loams) and practically inert in terms of corrosion (sandy loam, sandy soils).
The difference in the course of corrosion processes in different soils is also indicated by the British Standard BS 8004 “Foundations” (clause 10.3.5). In accordance with this document, the residual thickness of steel piles installed in undisturbed soils “remains within acceptable thickness values even after many decades of operation,” since the corrosion rate in these soils does not exceed 1-2 mm per 100 years. However, in disturbed soils, “the use of redox potential, soil resistivity, and pH values may have some value in predicting corrosion rates.” However, even in this case, the thickness of the metal should be selected based on the degree of aggressiveness of the disturbed soils.
Calculation formulas
In the general case, weight losses that determine the rate of metal corrosion are found using the following formula:
Vkp=q/(St),
where q is the reduction in metal mass, g;
S – surface area from which the material was transferred, m2;
t – time period, hours.
For sheet metal and shells made from it, the depth indicator is determined (mm/year):
H=m/t,
m is the depth of penetration of corrosion into the metal.
There is the following relationship between the first and second indicators described above:
H=8.76Vkp/ρ,
where ρ is the density of the material.
Classification of types of rust
Corrosion is classified according to the following criteria:
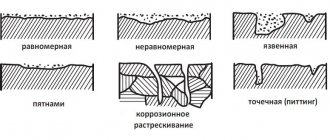
- According to the uniformity of flow. There is a more uniform, superficial corrosion (in which the wall thickness of the product decreases to the same degree) and uneven, focal corrosion, which is characterized by the appearance of damaged spots or ulcers on the steel surface.
- According to the direction of action. There is selective corrosion, in which only certain components of the metal structure are affected, and contact corrosion, which destroys a specific metal (for bimetallic compounds).
- In terms of the scale of their action, such types of corrosion are known as intercrystalline, destructively acting along the grain boundaries of steel (with gradual spread inward), and volumetric, affecting the entire surface simultaneously.
The intensity of corrosion increases significantly if, in addition to unfavorable changes/fluctuations in temperature and humidity, the contact surface of the metal is additionally affected by tensile stresses, as well as a chemically aggressive environment.
The intensity of corrosion increases many times due to cracking between adjacent crystallites and their blocks. External tensile-compressive stresses act on steel even more aggressively.
Main factors influencing the corrosion rate
You might be interested in: Comic nominations for teachers for graduation
The rate of metal destruction is influenced by the following groups of factors:
- internal, related to the physical and chemical nature of the material (phase structure, chemical composition, surface roughness of the part, residual and operating stresses in the material, and others);
- external (environmental conditions, speed of movement of a corrosive environment, temperature, atmospheric composition, presence of inhibitors or stimulants, and others);
- mechanical (development of corrosion cracks, destruction of metal under the influence of cyclic loads, cavitation and fretting corrosion);
- design features (choice of metal grade, presence of gaps between parts, roughness requirements).
Durability of a metal screw pile taking into account corrosion processes in the soil
One of the most significant issues that arise when using metal structures in construction is the issue of the resistance of such structures to corrosion processes and the associated durability of buildings and structures.
Currently, there is a set of interconnected interstate standards that establish general requirements, rules, norms and methods for protecting products, structures and materials from corrosion, aging and biological damage at all stages of the life cycle of products and structures, research and justification for development (ESZKS Standards - Unified System of Protection against corrosion and aging of materials and products) [1, 2, 3, 4].
The purpose of the ESZKS is to ensure and maintain a given level of quality of products, structures and materials using means and methods of protection against corrosion, aging and biodamage, taking into account the requirements of safety, ecology, compatibility and interchangeability, as well as the competitiveness of products and structures on the world market.
In addition to the ESZKS standards, requirements for corrosion resistance are also established by standards for certain types of structures and their parts, depending on the current corrosion factors.
For underground structures (including foundations), the corrosion hazard criteria are:
corrosive aggressiveness of the environment (soils, groundwater and other waters) in relation to the metal of the structure (including biocorrosive aggressiveness of soils);
dangerous effect of stray direct and alternating currents.
Based on these criteria, it follows that the rate of metal corrosion in the soil depends on:
Soil pH. The lower the pH (acidic environment), the higher the corrosion rate.
electrical resistance of soil a. The higher the soil resistance, the slower the corrosion rate.
It is also necessary to take into account the presence of an anti-corrosion coating that prevents corrosion.
Research to determine the electrical resistance of the soil, taking into account a possible increase in humidity and temperature changes, was carried out by the Federal Road Agency and is reflected in the manual for transport engineers (Table 1).
Source
Physicochemical characteristics
The most important among internal corrosion factors are the following:
- Thermodynamic stability. To determine it in aqueous solutions, Pourbaix reference diagrams are used, the abscissa of which is the pH of the medium, and the ordinate is the redox potential. A positive shift in potential means greater stability of the material. It is roughly defined as the normal equilibrium potential of the metal. In reality, materials corrode at different rates.
- Atom position in the periodic table of chemical elements. The metals most susceptible to corrosion are alkali and alkaline earth metals. The rate of corrosion decreases as the atomic number increases.
- Crystal structure. It has an ambiguous effect on destruction. The coarse-grained structure itself does not lead to an increase in corrosion, but is favorable for the development of intercrystalline selective destruction of grain boundaries. Metals and alloys with a uniform phase distribution corrode uniformly, while those with a non-uniform phase distribution corrode according to a focal mechanism. The mutual arrangement of the phases performs the function of anode and cathode in an aggressive environment.
- Energy heterogeneity of atoms in a crystal lattice. Atoms with the highest energy are located in the corners of the edges of microroughness and are active centers of dissolution during chemical corrosion. Therefore, careful mechanical processing of metal parts (grinding, polishing, finishing) increases corrosion resistance. This effect is also explained by the formation of denser and more continuous oxide films on smooth surfaces.
Information and analytical publication TECHNOmagazine
In chemistry there is the concept of “corrosion rate”. This is the depth of corrosion penetration into the metal per unit of time. For iron, the most typical indicator is 0.05-0.2 mm/year. This means that if the paintwork is damaged within 5 years, the thickness of the metal of the car body will decrease by 0.25-1 mm, if appropriate measures are not taken. In other words, in some places it will rust through.
Let's break it down
The problem of protecting metal from corrosion has faced humanity from the moment it learned about the existence of iron ore. There are documents from the 5th century BC. BC, where Herodotus describes the process of protecting an iron product by coating its surface with a layer of tin. And there is evidence from more ancient times about combating the effects of corrosion by lubricating surfaces with fat or various oils.
The word “corrosion” comes from the Latin “corrodo”, which means “to gnaw”. The definition, it should be noted, is clear and precise.
Corrosion is a very complex phenomenon. This is the process of spontaneous destruction of a metal as a result of oxidation, which is a consequence of chemical or electrochemical contact with the environment. Corrosion constantly affects vehicle parts, whether the equipment is running or is parked. In the latter case, corrosion is even much more active. The appearance of corrosion is always accompanied by an increase in the moisture-holding capacity of the metal, and the destructive process only accelerates. First of all, corrosion affects the metal of the cabin, frame surfaces, body parts, especially the metal of the exhaust manifold and exhaust system.
As a result of corrosion, an oxidized layer is formed on the metal surface. Some metals, such as aluminum, when corroded become covered with a dense oxide film that is well bonded to the metal. It is an excellent protection against further spread of corrosion into deeper layers of the metal, forming a kind of shield.
Iron is another matter. It rusts as a result of corrosion. This process is quite complex and has several stages. Iron hydroxide formed on the metal surface is a very unstable chemical compound. Losing water, it quickly turns into iron oxide. And this compound is not an obstacle to further oxidation. Therefore, iron parts that are not protected from corrosion can be completely destroyed quite quickly.
In chemical corrosion, metal destruction is caused by the action of dry gases, as well as contact with certain types of lubricants and organic coolants. This type of corrosion can be observed on the cylinder surfaces of carburetor and diesel engines, on the working surfaces of exhaust valves, and on the walls of combustion chambers. The phenomenon of chemical corrosion, caused by contact of metal with fuel and lubricants, manifests itself on the internal walls of fuel tanks.
Electrochemical corrosion occurs where metal comes into contact with moist gases, including atmospheric air. Also, this type of corrosion occurs at the point of contact between two dissimilar metals, creating a kind of galvanic couple. Under the influence of acids and salt solutions, an electrolytic process occurs, and the more active metal is quickly destroyed.
There is a division of types of corrosion from the point of view of corrosive environments.
Gas corrosion is chemical corrosion in a gas environment at high temperatures without the presence of moisture. Upon contact with a chemically active gas, a film of reaction products appears on the metal surface. At high temperatures, the process accelerates, the film thickens, preventing contact between the metal and the gas, and the metal surface intensively corrodes. This type of corrosion is a big problem for metallurgy and other industries where high temperatures and active gases are involved in the technology.
Underground corrosion involves corrosion of the metal upon contact with soil, while biocorrosion is characterized by the effect of microorganisms on the metal.
There is also radiation corrosion, which occurs under the influence of radioactive radiation. Corrosion caused by external and stray currents is distinguished.
There is corrosion that occurs due to the simultaneous influence of a corrosive environment and mechanical stress. This effect greatly activates the ongoing corrosion processes. In this case, oxide films on the metal surface may be damaged, electrochemical processes intensify in areas of heterogeneous metal composition, and microcracks may form. Corrosion that occurs during simultaneous impact and corrosive effects on metal is called cavitation.
All of the above types of corrosion are present to one degree or another in the “life” of automotive equipment, but the phenomena of electrochemical corrosion predominate.
Corrodes the underbody and inner surfaces of the wings. In general, all parts that come into contact with air and do not have a protective coating.
The fact is that after stamping and welding, fragments with a non-uniform structure appear in body parts. The base metal and impurities form many short-circuited microscopic galvanic cells. Slag inclusions and individual shells on the surface also contribute to the formation of galvanic couples, so steel body parts corrode intensively.
On the other hand, snowfalls, rains, and temperature changes contribute to the formation of a film of water condensate on the outer and inner walls of the body, ranging in thickness from several molecular layers to 1 mm. At a relative humidity of more than 60%, condensation is formed especially “productively”, and this level of humidity is present almost constantly in central Russia. Upon contact with atmospheric air, acids and alkalis penetrate into the condensate, coming from vehicle exhaust gases and harmful emissions into the atmosphere from various enterprises. And in winter, it also adds the possibility of connecting with the salt mixture that is sprinkled on our roads. An electrolyte is formed from the condensate with all the ensuing consequences for the metal of the machine.
By the way, also because of “anti-corrosion” measures, in Sweden and Norway they do not use any “chemicals” in the winter, but remove drifts with snow removal equipment, and in Finland in winter they sprinkle roads mainly with marble and granite chips.
Electrochemical corrosion is also called “atmospheric”. Science today determines more than 35 factors that influence the rate of atmospheric corrosion, such as the degree of metal moisture, the state of the metal surface (porosity, contamination), the chemical composition of the atmosphere (the presence of aggressive and hygroscopic products), etc.
If corrosion spreads over the entire surface of the metal, then it is called continuous. Moreover, a distinction is made between uniform corrosion, when the corrosion process occurs at the same speed over the entire surface, and uneven corrosion. Continuous corrosion spreads from the outer surface of the bottom, covering the wings, to the cabin parts.
Local corrosion affects individual areas of the metal surface. These could be rust spots that penetrate shallowly into the metal, or there could be spots (pittings) that go very deep into the metal structure. Such corrosion usually occurs in places where sheets are welded together, in places where the edges of the hood are rolled, and along the perimeter of the doors.
With selective corrosion in a metal that is an alloy of several elements, only one component corrodes, while the rest are not affected.
The phenomena of crevice corrosion occur in places of threaded connections, in gaps between loosely fitting parts, in places where gaskets do not fit tightly.
But the most dangerous, perhaps, are subsurface (subfilm) and intercrystalline corrosion. Subsurface corrosion causes delamination of metal sheets and products; in this case, corrosion products become concentrated in metal cavities formed as corrosion spreads deeper into the metal. And intercrystalline corrosion is expressed in the destruction of metal along grain boundaries. The metal loses strength, ductility, and becomes brittle, although the appearance of the surface is quite normal.
Practical actions in the fight against this insidious phenomenon
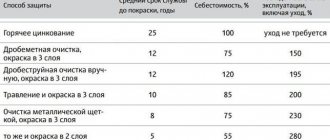
When assembling modern vehicles, parts and assemblies are protected from corrosion using paint, plastic, galvanic and chemical coatings, as well as preservative oils.
Also, to increase corrosion resistance, the surface of iron and steel products is coated with deposits of other metals. In addition to simply mechanical insulation of the base surface, the electrochemical properties change.
To reduce the rate of corrosion, steel is coated with copper, nickel, and rhodium using the anodic protection method. For the same purpose, to reduce the rate of “rusting,” the metal is coated with lead, tin, and zinc, and the polarization of the cathode reaction increases.
Body parts are most often galvanized. An electric couple Zinc-Steel arises, where zinc, intensively oxidizing, but incomparably slower than iron, protects the steel.
When processed by the hot galvanizing method, which consists of immersing a part or structure in a bath of molten zinc, a corrosion-free service life of steel structures is ensured, depending on the thickness of the coating layer, from 10 to 50 years. Thermal diffusion galvanizing gives the product up to half a century of corrosion-free service. In this case, when heated to 350-420° C, zinc is introduced into the surface layer of the metal with the formation of chemical compounds - Zn-Fe intermetallic compounds of various compositions.
The most effective way to protect a structure from corrosion is the use of alloyed materials. The corrosion resistance of steel increases sharply when only a few percent of chromium is added to the alloy. To increase, including corrosion resistance, the frames of Turkish-made Ford Cargo trucks are made of high-strength steel with niobium additives.
In general, if we talk about foreign-made trucks (not Chinese ones), what attracts attention is the high quality of painting, soldering, and seam processing. All connections of individual elements are sealed and carefully coated with special compounds. The body and underbody are usually treated with cataphoresis priming. Such factory corrosion protection instills confidence in the long “life” of the machine’s metal. Domestic equipment is not always so thoroughly prepared when leaving the factory, and the problem of corrosion protection is shifted to consumers.
You can still resist!
Keeping both commercial vehicles and special equipment clean is one of the prerequisites for the fight against corrosion. A dirty car is subject to increased corrosion and destruction of the paintwork. It is under the layer of dirt that a damp atmosphere ideal for rust is created. And no anti-corrosion agents will help when the equipment is not cleaned, that is, it is constantly exposed to an aggressive environment consisting of a mixture of dust, sand, water, air, oil products and reagents. During operation, only high-quality oils and fluids from well-known manufacturers should be used. Automotive oils, both motor, transmission and hydraulic, must contain a sufficient amount of anti-corrosion additives. The coolant must also contain anti-corrosion components, which are designed for a long service life. Modern technologies based on the use of aliphatic acids as anti-corrosion additives provide long-term protection against destruction of all metals, including aluminum and alloys present in the engine, and the properties of inhibitory (slowing down corrosion processes) additives last for at least 5 years. Considering the huge number of counterfeit products on the motor transport market, coolant should only be purchased from “trusted” suppliers.
(end to follow)
Nikolay Dneprov
Author: TECHNOmagazine
Share
Influence of acidity of the environment
You might be interested in: Dig or drip? How to write correctly?
During the process of chemical corrosion, the concentration of hydrogen ions affects the following points:
- solubility of corrosion products;
- formation of protective oxide films;
- metal destruction rate.
At a pH in the range of 4-10 units (acidic solution), corrosion of iron depends on the intensity of oxygen penetration to the surface of the object. In alkaline solutions, the corrosion rate first decreases due to surface passivation, and then, at pH>13, increases as a result of the dissolution of the protective oxide film.
Each type of metal has its own dependence of the intensity of destruction on the acidity of the solution. Noble metals (Pt, Ag, Au) are resistant to corrosion in acidic environments. Zn and Al are quickly destroyed in both acids and alkalis. Ni and Cd are resistant to alkalis, but easily corrode in acids.
Mechanisms of occurrence and development of corrosion phenomena
Since most steel surfaces operate in an environment of certain humidity, as well as in water, aqueous solutions of salts, acids and alkalis, the predominant mechanism of rust formation is electrolytic. The only exception is furnace corrosion, which occurs in metal structures of heating devices: there, surface destruction occurs due to the formation of high-temperature rust - scale.
Electrolytic
During electrolytic corrosion in the presence of oxygen, a hydration reaction of steel iron occurs, the final product of which is iron oxide hydrate Fe(OH)2. This phenomenon is called anodic type corrosion. But the process does not end there. Iron oxide hydrate is an unstable substance and, in the presence of water (or water vapor), quite quickly decomposes into various iron oxides:
- at elevated temperatures, iron oxide FeO is formed predominantly;
- at room temperature or slightly higher – iron oxide Fe2O3;
- at intermediate temperatures (in the temperature range +250...+450°C) – magnetic iron oxide Fe3O4.
In any case, the surface of the steel rusts, only indicators of this phenomenon can be either reddish-brown or grayish-yellow.
In the presence of acids
A slightly different mechanism of rust formation occurs in the presence of acids, acidic solutions or liquid media that do not contain oxygen. Here, anodic dissolution of steel occurs with the formation of hydrides - compounds of iron with hydrogen. But the latter are chemically unstable substances, quickly oxidize in an airy and humid environment and also form rust, only more friable. Iron hydrides decompose especially quickly when sulfur compounds are present in the atmosphere or environment.
In the presence of loads
According to the third scheme, corrosion occurs when external loads are applied to the contact surfaces. Here, in addition to the two traditional components, a third component is necessarily present - lubricant. Since all organic compounds always contain oxygen and hydrogen, when the temperature rises at the contact, mechanochemical reactions of lubricant oxidation begin to occur. They end with the fact that, instead of reducing friction, the used and partially destroyed lubricant begins to actively oxidize the surfaces, forming rust.
Composition and concentration of neutral solutions
The rate of corrosion in neutral solutions depends to a large extent on the properties of the salt and its concentration:
- The hydrolysis of salts in a corrosive environment produces ions that act as activators or retarders (inhibitors) of metal destruction.
- Those compounds that increase pH also increase the rate of the destructive process (for example, soda ash), and those that reduce acidity reduce it (ammonium chloride).
- In the presence of chlorides and sulfates in the solution, destruction is activated until a certain salt concentration is reached (which is explained by the intensification of the anodic process under the influence of chlorine and sulfur ions), and then gradually decreases due to a decrease in oxygen solubility.
Some types of salts are capable of forming a poorly soluble film (for example, iron phosphate). This helps protect the metal from further destruction. This property is used when using rust neutralizers.
Corrosion inhibitors
Corrosion retarders (or inhibitors) differ in their mechanism of action on the redox process:
- Anode. Thanks to them, a passive film is formed. This group includes compounds based on chromates and dichromates, nitrates and nitrites. The last type of inhibitors is used for inter-operational protection of parts. When using anodic corrosion inhibitors, it is necessary to first determine their minimum protective concentration, since addition in small quantities can lead to an increase in the rate of destruction.
- Cathode. The mechanism of their action is based on reducing the oxygen concentration and, accordingly, slowing down the cathodic process.
- Shielding. These inhibitors isolate the metal surface by forming insoluble compounds that are deposited as a protective layer.
The last group includes rust neutralizers, which are also used to remove oxides. They usually contain orthophosphoric acid. Under its influence, phosphating of the metal occurs - the formation of a durable protective layer of insoluble phosphates. Neutralizers are applied with a spray or roller. After 25-30 minutes the surface becomes white-gray. After the composition has dried, paint and varnish materials are applied.
Mechanical impact
Increased corrosion in an aggressive environment is facilitated by such types of mechanical impact as:
- Internal (during molding or heat treatment) and external (under the influence of externally applied load) stresses. As a result, electrochemical heterogeneity occurs, the thermodynamic stability of the material decreases, and corrosion cracking occurs. Destruction occurs especially quickly under tensile loads (cracks form in perpendicular planes) in the presence of oxidizing anions, for example, NaCl. A typical example of devices susceptible to this type of destruction are parts of steam boilers.
- Alternating dynamic effects, vibrations (corrosion fatigue). There is an intensive decrease in the fatigue limit, multiple microcracks are formed, which then merge into one large one. The number of cycles before failure largely depends on the chemical and phase composition of metals and alloys. Pump axles, springs, turbine blades and other equipment elements are susceptible to such corrosion.
- Friction of parts. Rapid corrosion is caused by mechanical wear of protective films on the surface of the part and chemical interaction with the environment. In liquid, the rate of destruction is lower than in air.
- Cavitation impact. Cavitation occurs when the continuity of fluid flow is disrupted as a result of the formation of vacuum bubbles, which collapse and create a pulsating effect. As a result, deep local damage occurs. This type of corrosion is often observed in chemical apparatus.
Design factors
When designing elements operating in aggressive conditions, it must be taken into account that the corrosion rate increases in the following cases:
- upon contact of dissimilar metals (the greater the difference in electrode potential between them, the higher the current strength of the electrochemical destruction process);
- in the presence of mechanical stress concentrators (grooves, grooves, holes, etc.);
- with low cleanliness of the treated surface, since in this case local short-circuited galvanic pairs arise;
- when there is a significant difference in the temperature of individual parts of the apparatus (thermogalvanic elements are formed);
- in the presence of stagnant zones (cracks, gaps);
- during the formation of residual stresses, especially in welded joints (to eliminate them, it is necessary to provide heat treatment - annealing).
Assessment methods
There are several ways to assess the rate of destruction of metals in aggressive environments:
- Laboratory - testing samples in artificially simulated conditions close to real ones. Their advantage is that they can reduce research time.
- Field – carried out in natural conditions. They take a long time. The advantage of this method is to obtain information about the properties of the metal under further operating conditions.
- Full-scale – testing of finished metal objects in a natural environment.
Source
Durability of a metal screw pile taking into account corrosion processes in the soil
Corrosion rate is a multifactorial parameter that depends on both external environmental conditions and the internal properties of the material. In the regulatory and technical documentation there are certain restrictions on the permissible values of metal destruction during the operation of equipment and building structures to ensure their trouble-free operation. In design, there is no universal method for determining corrosion rate. This is due to the complexity of taking into account all factors. The most reliable method is to study the operating history of the facility.