Despite the widespread use of plastic, metal pipelines are still widely used for transporting acids, alkalis, gases, petroleum products, etc. Such structures begin to deteriorate over time due to atmospheric, chemical and other types of corrosion. Although this is a natural process, it can nevertheless be slowed down. This is why there is protective protection of metal from corrosion.
Causes of corrosion
The development of pipeline corrosion occurs as a result of the oxidation reaction of the metal from constant exposure to a humid environment. The composition of the metal changes at the ionic level. This process can be influenced by the composition of the liquid flowing inside the pipeline. The causes of rust may be the following:
- The alloys from which pipelines are made have different electrochemical potentials. This causes currents to flow through the pipe. Different potentials can arise due to changes in soil components, as well as different parameters of environmental indicators.
- Groundwater or moisture found in the soil.
- The chemical composition of the soil, including the presence of acidic impurities in the external environment.
- Composition of the liquid transported by the pipeline.
- The presence of stray currents in the soil.
To perform anti-corrosion protection, it is necessary to evaluate the characteristics affecting the metal surface.
The essence of electrochemical protection
A direct current (DC source or protector) is connected to the finished metal product from the outside. Electric current on the surface of the protected product creates cathodic polarization of the electrodes of microgalvanic pairs. The result of this is that the anodic areas on the metal surface become cathodic. And due to the influence of a corrosive environment, it is not the metal of the structure that is destroyed, but the anode.
Depending on which direction (positive or negative) the metal potential shifts, electrochemical protection is divided into anodic and cathodic.
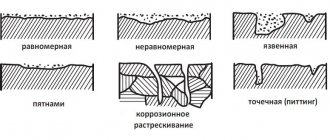
About types of corrosion
There are several types of corrosion of metal pipes:
- superficial, spreading over the entire area of the pipe;
- local, located in separate areas;
- crevice formed in a small crack.
Local corrosion is the most alarming, since the bulk of damage occurs as a result of its occurrence. The development of crevice is also common, but it does not lead to significant damage to the material.
The probability of corrosion is higher for sections of pipes extended under railway crossings or under overhead power line supports. The rate of development of the corrosion process ranges from 3 to 30 mm per year.
What is chemical corrosion
This process occurs in non-electrically conductive media. They may be gases, petroleum products and alcohol compounds. As temperatures increase, the rate of corrosion development increases. Rust can form on non-ferrous or ferrous metals. Aluminum products under the influence of corrosive factors are covered with a thin film, which then provides a protection system and creates an obstacle to the development of the oxidation process.
Under the influence of this type of corrosion, copper begins to turn green, while the formed oxide film in a humid environment does not always contribute to the creation of a protective barrier against rust, but only as an exception, when the structure of the metal is the same as the structure of the film.
Alloys may be susceptible to another type of rust, that is, there are elements that are not subject to oxidation, but on the contrary, they are reduced. For example, with increased temperature characteristics and increased pressure, carbides are restored, but, again, the necessary qualities are lost.
About electrochemical corrosion
The statement that electrochemical corrosion is achieved only when a metal surface comes into contact with an electrolyte is erroneous. A thin film on the base of the material is enough for corrosion to form. The cause of this type of rust is the use of table or industrial salts. For example, if snow is spread on the roads, then cars and pipelines laid underground suffer.
The process of this origin is as follows:
- In the connections of metal structures, atoms are partially lost, they are transferred into an electrolytic solution, that is, ions are formed. Atoms replace electrons, they charge the material with negative charges, while positive charges accumulate in the electrolyte.
- Electrochemical corrosion is also caused by stray currents, which, when leaking from an electrical circuit, go into water solutions or into the soil, and then into the metal structure itself. Specific places where rust appears are those areas from which stray currents enter the water.
On video: electrochemical corrosion of metals and methods of protection.
How to provide protective protection
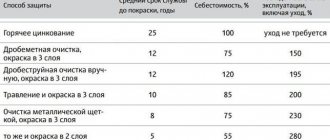
Coating pipes with special compounds is not only a task for the manufacturer; during the operation of the structure, ensuring protective properties must also be carried out. There are several ways to protect metal from aggressive environments:
- chemical treatment;
- coating the walls with special compounds;
- protection against stray currents;
- connecting the cathode or anode.
The method of tread protection of pipelines from corrosion is popular in organizations that install and operate pipeline transport.
About passive and active methods
Anti-corrosion protection is a whole range of measures carried out by enterprises. Passive protection methods involve performing the following work:
- At the installation stage, an air gap is left between the pipeline and the ground to prevent the ingress of groundwater, including those containing acidic and alkaline impurities.
- Coating with specialized compounds, the purpose of which extends from the negative effects of the soil.
- Treatment of metal with chemical compounds to form a thin film.
Active methods of protection involve the use of current and ion exchange based on chemical reactions, which ensures:
- Protection of underground pipelines from corrosion by creating an electrical drainage system to isolate pipeline transport from stray currents.
- Anode protection against destruction of metal surfaces.
- Cathodic protection to increase the resistance of metal substrates.
Only by taking into account all the methods that prevent the formation of rust on metal will the service life of structures be increased. Anti-corrosion protection of pipelines must be carried out comprehensively.
On video: protecting pipelines and cable lines from electrical corrosion.
About the advantages of using protectors
Pipe protection in this way is carried out with the addition of a component - an inhibitor. It is a material with a negative electrical charge. Under the influence of air masses it dissolves, but the structure remains intact and does not rust. Tread protection against corrosion is used to extend the service life of building structures, heating and water supply systems, as well as main and field pipeline transport.
The use of electrochemical protection makes it possible to eliminate the causes of many types of corrosion. Such anti-corrosion protection of pipelines is a good solution even for enterprises that do not have the financial capacity to provide full protection against an uncontrolled process.
To ensure a competent approach you should:
- Protectors made of aluminum are used in marine environments and coastal shelves.
- In environments with low electrical conductivity, use magnesium protectors. But, again, they are not suitable for treating the internal coating of tanks and oil settling tanks due to the fact that they have a fairly low fire and explosion hazard.
- Use protectors to protect against fresh water environments.
- Projectors made on the basis of zinc are completely safe and can be used in fire and explosion hazardous industries.
Protective anti-corrosion protection includes the following number of advantages:
- the lack of funds and production capacity of the enterprise will not be an obstacle to its implementation;
- the ability to protect small structures;
- if the pipes are covered with thermal insulation materials, then such protection is acceptable.
Materials used and purposes of application
Anti-corrosion protection is required for all metal substrates. This type of anti-rust treatment is widely used for treating tankers, since these vessels are most susceptible to exposure to water containing aggressive components. Even special painting cannot solve this problem.
The most rational choice for coating steel structures would be to use protectors with negative potential. In the manufacture of such devices, magnesium, zinc or aluminum is used. The large difference in potential between metal and steel surfaces helps to increase the spectrum of protective action, as a result, various types of corrosion are eliminated.
The protection system is carried out based on the specifics of the protectors themselves, as well as the environments in which they will be used.
Passive protection is required for steel coatings and metal products. The essence of the method is the use of galvanic anodes, which ensure that underground pipelines resist corrosion. When making calculations for this installation, the following indicators must be taken into account:
- current parameters;
- resistance against voltage surges;
- characteristics of the degree of protection used for 1 km of pipeline;
- indicator of the distance between protection elements.
Combination of protectors and paints
Often there is a need to protect a gas pipeline from corrosion not only with a protector, but with paint and varnish material. Paint is considered a passive method of protection against corrosion processes and is truly effective only when combined with the use of a protector.
This combination technique allows:
- Reduce the negative impact of potential defects in the coating of metal structures (peeling, swelling, cracking, heaving, etc.). Such defects occur not only as a result of manufacturing defects, but also due to natural factors.
- Reduce (sometimes by a very significant amount) the consumption of expensive protectors, while increasing their service life.
- Make the distribution of the protective layer over the metal more uniform.
It is also worth noting that paint and varnish compositions are often not easy to apply to certain surfaces of an already operating gas pipeline, tanker or some other metal structure. In such cases, you will have to make do with only a protective protector.
Methods for protecting pipelines
Corrosion of pipelines occurs during their operation. Rust formation occurs on pipes inside and outside. Deposits appear on the inside, and the reason for this is chemical reactions between the composition of the transported liquid and the metal. The condition of the surface is also influenced by high soil moisture.
If protection is not provided in a timely manner, a number of consequences may occur. What is important:
- It is recommended to carry out routine inspections at short intervals.
- Carry out repair work periodically, regardless of the presence of corrosion.
- suspension of the functioning of pipeline transport is inevitable, since it is necessary to carry out inspections and carry out scheduled preventative and other routine repairs.
Important! To ensure complete protection, it is necessary to take into account the installation method, contact with aggressive media, and the type of pipeline.