Oxides are a widespread type of compound in nature, which can be observed even in everyday life. Examples include sand, water, rust, lime, carbon dioxide, and a number of natural dyes. The ore of many valuable metals is an oxide in nature, which is why it is of great interest for scientific and industrial research.
The combination of chemical elements with oxygen is called oxides. As a rule, they are formed when any substances are heated in air. There are acidic and basic oxides. Metals form basic oxides, while non-metals form acidic oxides. With the exception of chromium and manganese oxides, which are also acidic. This article discusses a representative of the main oxides - CuO (II).
Brief characteristics of copper (II) oxide:
Copper (II) oxide is a black inorganic substance.
Since the valence of copper varies and is equal to one, two or three, copper oxide contains, respectively, two copper atoms and one oxygen atom, one copper atom and one oxygen atom, two copper atoms and three oxygen atoms.
Cuprous oxide contains one copper atom and one oxygen atom, respectively.
The chemical formula of copper (II) oxide is CuO.
Powder. Does not dissolve in water.
Chemical Formula: CuO
Its molecule consists of a Cu atom with a molecular weight of 64 a. e.m. and O atom, molecular weight 16 a. e.m., where a. e.m. - atomic unit of mass, also known as dalton, 1 a. e.m. = 1.660 540 2(10) × 10−27 kg = 1.660 540 2(10) × 10–24 g. Accordingly, the molecular weight of the compound is: 64 + 16 = 80 a. eat.
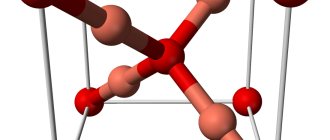
Crystal lattice: monoclinic system. What does this type of crystal symmetry axes mean when two axes intersect at an oblique angle and have different lengths, and the third axis is located at an angle of 90° with respect to them.
Density 6.51 g/cm3. For comparison, the density of pure gold is 19.32 g/cm³, and the density of table salt is 2.16 g/cm³.
Melts at a temperature of 1447 °C , under oxygen pressure.
Decomposes when heated to 1100 °C and is converted to copper (I) oxide:
4CuO = 2Cu2O + O2.
Does not react with water and does not dissolve in it.
But it reacts with an aqueous solution of ammonia to form tetraammine copper (II) hydroxide: CuO + 4NH3 + H2O = [Cu (NH3)4](OH) 2.
In an acidic environment it forms sulfate and water: CuO + H2SO4 = CuSO4 + H2O.
Reacting with alkali, it creates cuprate: CuO + 2 NaOH → Na2CuO2 + H2O.
Physical properties of copper (II) oxide:
| Parameter name: | Meaning: |
| Chemical formula | CuO |
| Synonyms and names in a foreign language | copper oxide (outdated Russian) copper (II) oxide tenorite (Russian) |
| Type of substance | inorganic |
| Appearance | black powder |
| Color | black |
| Taste | —* |
| Smell | — |
| Physical state (at 20 °C and atmospheric pressure 1 atm.) | solid |
| Density (state of matter – solid, at 20 °C), kg/m3 | 6310 |
| Density (state of matter – solid, at 20 °C), g/cm3 | 6,31 |
| Boiling point, °C | 2000 |
| Melting point, °C | 1447 |
| Decomposition temperature, °C | 800 |
| Molar mass, g/mol | 79,545 |
*Note:
- no data.
Experiment 2. Determination of carbon and hydrogen by burning a substance with copper (II) oxide
Reagents and materials: glucose (or glycerin, starch, sugar); copper oxide (powder); barite water, saturated aqueous solution; Copper (II) sulfate, anhydrous. Equipment: gas outlet tube with plug; glass rod; micro-spatula.
into dry test tube 1 (Fig. ). Add half a microspatula of glucose and mix thoroughly, shaking the test tube. A small ball of cotton wool is placed in the upper part of the test tube, on which a little white anhydrous copper sulfate powder is poured ( II) The test tube is closed with a stopper with a gas outlet tube. In this case, the end of the tube should almost rest against the cotton wool with CuSO4.
The lower end of the tube is lowered into test tube 2,
first pour 5-6 drops of barite (or lime) water into it. Test tube 1 is heated on a flame
burners.
After a few seconds, gas bubbles begin to emerge from the gas outlet tube, and the barite water becomes cloudy due to the release of a white precipitate of barium carbonate. Test tube 2 is removed. Continue heating test tube 1 ,
until water vapor reaches the white powder of dehydrated copper sulfate located on the cotton plug and causes a change in its color due to the formation of crystalline hydrate CuSO4*5H2O.
Process chemistry:
C6H12O6 + 12CuO → 6CO2 + 6H2O + 12Cu
CO2 + Ba(OH)2 → BaCO3 + H2O
CuSO4 + 5H2O → CuSO4*5H2O
The method is based on the fact that when an organic substance is calcined in a mixture with an oxidizing agent (CuO), the carbon of the organic substance is oxidized into carbon dioxide, and hydrogen into water. Copper oxide is reduced to copper metal.
The release of carbon dioxide is evidenced by the appearance of a white precipitate of barium carbonate. Water in combustion products is detected by the formation of blue crystals of copper sulfate.
Reagents and materials: glucose (or glycerin, starch, sugar); copper oxide (powder); barite water, saturated aqueous solution; Copper (II) sulfate, anhydrous. Equipment: gas outlet tube with plug; glass rod; micro-spatula.
into dry test tube 1 (Fig. ). Add half a microspatula of glucose and mix thoroughly, shaking the test tube. A small ball of cotton wool is placed in the upper part of the test tube, on which a little white anhydrous copper sulfate powder is poured ( II) The test tube is closed with a stopper with a gas outlet tube. In this case, the end of the tube should almost rest against the cotton wool with CuSO4.
The lower end of the tube is lowered into test tube 2,
first pour 5-6 drops of barite (or lime) water into it. Test tube 1 is heated on a flame
burners.
After a few seconds, gas bubbles begin to emerge from the gas outlet tube, and the barite water becomes cloudy due to the release of a white precipitate of barium carbonate. Test tube 2 is removed. Continue heating test tube 1 ,
until water vapor reaches the white powder of dehydrated copper sulfate located on the cotton plug and causes a change in its color due to the formation of crystalline hydrate CuSO4*5H2O.
Process chemistry:
C6H12O6 + 12CuO → 6CO2 + 6H2O + 12Cu
CO2 + Ba(OH)2 → BaCO3 + H2O
CuSO4 + 5H2O → CuSO4*5H2O
The method is based on the fact that when an organic substance is calcined in a mixture with an oxidizing agent (CuO), the carbon of the organic substance is oxidized into carbon dioxide, and hydrogen into water. Copper oxide is reduced to copper metal.
The release of carbon dioxide is evidenced by the appearance of a white precipitate of barium carbonate. Water in combustion products is detected by the formation of blue crystals of copper sulfate.
Preparation of copper (II) oxide:
Copper (II) oxide is obtained as a result of the following chemical reactions:
1. copper oxidation:
2Cu + O2 → CaO.
2. thermal decomposition of copper ( II ) hydroxide, copper ( II ) nitrate, copper carbonate ( II ):
Cu(OH)2 → CuO + H2O (to);
2Cu(NO3)2 → 2CuО + 4NO2 + O2 (to);
CuCO3 → CuO + CO2 (to).
3. heating malachite:
Cu2CO3(OH)2 → 2CuO + CO2 + H2O (to).
Reaction CuO NaOH
Formed:
- by calcining copper (II) hydroxide at a temperature of 200 °C: Cu(OH)2 = CuO + H2O;
- during the oxidation of copper metal in air at a temperature of 400–500 °C: 2Cu + O2 = 2CuO;
- during high-temperature processing of malachite: (CuOH)₂CO₃ —> 2CuO + CO₂ + H₂O.
Reduced to metallic copper -
- in reaction with hydrogen: CuO + H2 = Cu + H2O;
- with carbon monoxide (carbon monoxide): CuO + CO = Cu + CO2;
- with active metal: CuO + Mg = Cu + MgO.
Toxic . Based on the degree of adverse effects on the human body, it is classified as a substance of the second hazard class. Causes irritation to the mucous membranes of the eyes, skin, respiratory tract and gastrointestinal system. When interacting with it, it is necessary to use protective equipment such as rubber gloves, respirators, safety glasses, and special clothing.
The substance is explosive and flammable.
It is used in industry as a mineral component of mixed feed, in pyrotechnics, in the production of catalysts for chemical reactions, as a coloring pigment for glass, enamels, and ceramics.
The oxidizing properties of copper (II) oxide are most often used in laboratory research when elemental analysis is needed to study organic materials for the presence of hydrogen and carbon.
It is important that CuO (II) is quite widespread in nature, like the mineral tenerite, in other words, it is a natural ore compound from which copper can be obtained.
The Latin name Cuprum and its corresponding symbol Cu come from the name of the island of Cyprus. It was from there, across the Mediterranean Sea, that the ancient Romans and Greeks exported this valuable metal.
Copper is one of the seven most common metals in the world and has been in the service of humans since ancient times. However, in its original, metallic state it is quite rare. This is a soft, easy-to-process metal, characterized by high density, and a very high-quality conductor of current and heat. In electrical conductivity it is second only to silver, while being a cheaper material. Widely used in the form of wire and thin sheets.
Chemical compounds of copper are characterized by increased biological activity. In animal and plant organisms they participate in the processes of chlorophyll synthesis, therefore they are considered a very valuable component in mineral fertilizers.
Copper is also necessary in the human diet. Its lack in the body can lead to various blood diseases.
Chemical properties of copper (II) oxide. Chemical reactions of copper (II) oxide:
Copper (II) oxide is a basic oxide.
The chemical properties of copper(II) oxide are similar to those of basic oxides of other metals. Therefore, it is characterized by the following chemical reactions:
1. reaction of copper (II) with hydrogen:
CuO + H2 → Cu + H2O (t = 300 oC).
The reaction produces copper and water.
2. reaction of copper (II) oxide with carbon:
CuO + C → Cu + CO (t = 1200 oC).
The reaction produces copper and carbon monoxide.
3. reaction of copper (II) with sulfur:
CuO + 2S → Cu + S2O (t = 150-200 oC).
The reaction takes place in a vacuum. As a result of the reaction, copper and sulfur oxide are formed.
4. reaction of copper (II) with aluminum:
3CuO + 2Al → 3Cu + Al2O3 (t = 1000-1100 oC).
As a result of the reaction, copper and aluminum oxide are formed.
5. reaction of copper (II) with copper:
CuO + Cu → Cu2O (t = 1000-1200 oC).
As a result of the reaction, copper (I) oxide is formed.
6. reaction of copper (II) with lithium oxide:
CuO + Li2O → Li2CuO2 (t = 800-1000 oC, O2).
The reaction takes place in a flow of oxygen. As a result of the reaction, lithium cuprate is formed.
7. reaction of copper (II) with sodium oxide:
CuO + Na2O → Na2CuO2 (t = 800-1000 oC, O2).
The reaction takes place in a flow of oxygen. As a result of the reaction, sodium cuprate is formed.
8. reaction of copper (II) with carbon monoxide:
CuO + CO → Cu + CO2.
The reaction produces copper and carbon monoxide (carbon dioxide).
9. reaction of copper (II) with iron oxide:
CuO + Fe2O3 → CuFe2O4 (to).
As a result of the reaction, a salt is formed - copper ferrite. The reaction occurs when the reaction mixture is calcined.
10. reaction of copper (II) with hydrofluoric acid:
CuO + 2HF → CuF2 + H2O.
As a result of a chemical reaction, a salt is obtained - copper fluoride and water.
11. reaction of copper (II) with nitric acid:
CuO + 2HNO3 → 2Cu(NO3)2 + H2O.
As a result of a chemical reaction, salt is obtained - copper nitrate and water.
(II) with other acids proceed similarly
12. reaction of copper (II) with hydrogen bromide (hydrogen bromide):
CuO + 2HBr → CuBr2 + H2O.
As a result of a chemical reaction, salt is obtained - copper bromide and water.
13. reaction of copper (II) with hydrogen iodide:
CuO + 2HI → CuI2 + H2O.
As a result of a chemical reaction, salt is obtained - copper iodide and water.
14. reaction of copper (II) with sodium hydroxide:
CuO + 2NaOH → Na2CuO2 + H2O.
As a result of a chemical reaction, salt is obtained - sodium cuprate and water.
15. reaction of copper (II) with potassium hydroxide:
CuO + 2KOH → K2CuO2 + H2O.
As a result of a chemical reaction, salt is obtained - potassium cuprate and water.
16. reaction of copper (II) with sodium hydroxide and water:
CuO + 2NaOH + H2O → Na2[Cu2(OH)]2 (t = 100 oC).
Sodium hydroxide is dissolved in water. A solution of sodium hydroxide in water 20-30%. The reaction occurs at boiling point. As a result of a chemical reaction, sodium tetrahydroxycuprate is obtained.
17. reaction of copper (II) with potassium superoxide:
2CuO + 2KO2 → 2KCuO2 + O2 (t = 400-500 oC).
As a result of a chemical reaction, a salt is obtained - potassium cuprate (III) and oxygen.
18. reaction of copper (II) with potassium peroxide:
2CuO + 2K2O2 → 2KCuO2 (t = 700 oC).
As a result of a chemical reaction, a salt is obtained - potassium cuprate (III).
19. reaction of copper (II) with sodium peroxide:
2CuO + 2Na2O2 → 2NaCuO2 (t = 700 oC).
As a result of a chemical reaction, a salt is obtained - sodium cuprate (III).
20. reaction of copper (II) with ammonia:
3CuO + 2NH3 → N2 + 3Cu + 3H2O (t = 500-550 oC).
Ammonia is passed through heated copper(II) oxide. The chemical reaction produces nitrogen, copper and water.
6CuO + 4NH3 → 2Cu3N + N2 + 6H2O (t = 250-300 oC).
As a result of a chemical reaction, copper nitride, nitrogen and water are obtained.
21. reaction of copper (II) oxide and aluminum iodide:
6CuO + 4AlI3 → 6CuI + 2Al2O3 + 3I2 (t = 230 oC).
As a result of a chemical reaction, salt is obtained - copper iodide, aluminum oxide and iodine.
Copper oxide (I, II, III): properties, preparation, application
As you know, in chemistry there are four classes of inorganic compounds. There are a lot of substances representing each of them, but the leading position is undoubtedly occupied by oxides. One chemical element can have several different binary compounds with oxygen at once. Copper also has this property. It has three oxides. Let's look at them in more detail.
Copper(I) oxide
Its formula is Cu2O. In some sources, this compound may be called copper hemioxide, dicopper oxide or cuprous oxide.
Properties
It is a crystalline substance with a brown-red color. This oxide is insoluble in water and ethyl alcohol. It can melt without decomposing at a temperature slightly above 1240°C. This substance does not interact with water, but can be transferred into solution if the participants in the reaction with it are concentrated hydrochloric acid, alkali, nitric acid, ammonia hydrate, ammonium salts, and sulfuric acid.
Preparation of copper(I) oxide
It can be obtained by heating copper metal, or in an environment where oxygen has a low concentration, as well as in a flow of certain nitrogen oxides and together with copper (II) oxide. In addition, it can become a product of the thermal decomposition reaction of the latter. Copper (I) oxide can also be obtained if copper (I) sulfide is heated in a stream of oxygen. There are other, more complex ways to obtain it (for example, reduction of one of the copper hydroxides, ion exchange of any monovalent copper salt with alkali, etc.), but they are practiced only in laboratories.
Application
Needed as a pigment when painting ceramics and glass; a component of paints that protect the underwater part of a vessel from fouling. Also used as a fungicide. Copper oxide valves cannot do without it.
Copper(II) oxide
Its formula is CuO. In many sources it can be found under the name copper oxide.
Properties
It is a higher oxide of copper. The substance has the appearance of black crystals that are almost insoluble in water. It reacts with acid and during this reaction forms the corresponding cupric salt, as well as water. When it is fused with alkali, the reaction products are cuprates. The decomposition of copper (II) oxide occurs at a temperature of about 1100°C. Ammonia, carbon monoxide, hydrogen and coal are capable of extracting copper metal from this compound.
Receipt
It can be obtained by heating copper metal in an air environment under one condition - the heating temperature must be below 1100°C. Also, copper (II) oxide can be obtained by heating carbonate, nitrate, and divalent copper hydroxide.
Application
Using this oxide, enamel and glass are colored green or blue, and a copper-ruby variety of the latter is also produced. In the laboratory, this oxide is used to detect the reducing properties of substances.
Copper(III) oxide
Its formula is Cu2O3. It has a traditional name, which probably sounds a little unusual - copper oxide.
Properties
It looks like red crystals that do not dissolve in water. The decomposition of this substance occurs at a temperature of 400 ° C, the products of this reaction are copper (II) oxide and oxygen.
Receipt
It can be prepared by oxidizing copper hydroxide with potassium peroxydisulfate. A necessary condition for the reaction is an alkaline environment in which it must occur.
Application
This substance is not used by itself. In science and industry, its decomposition products—copper (II) oxide and oxygen—are more widely used.
Conclusion
That's all copper oxides. There are several of them due to the fact that copper has a variable valence. There are other elements that have several oxides, but we’ll talk about them another time.
Crystal lattice:
| 300 | Crystal cell |
| 311 | Crystal grid #1 |
| 312 | Lattice structure |
| 313 | Lattice parameters |
| 314 | c/a ratio |
| 315 | Debye temperature |
| 316 | Name of space symmetry group |
| 317 | Symmetry space group number |