Dealing with rust and not damaging the metal surface is not an easy task.
The mechanical method of removing it leads to scratches on the product, and chemical reagents provoke oxidation processes, as a result of which the part will rust even more in the future.
A safe and relatively simple way to combat rust is to remove it by electrolysis. Read the article to learn how effective this method is and how to implement it correctly in practice.
Is the method effective?
Electrolysis really helps with rust. It is a mixture of iron oxides and hydroxides, which are formed when the metal comes into contact with water and oxygen.
Electrolysis is a chemical reaction that reduces iron from its oxide to its metallic form. Wherever the electrolyte penetrates, the process of rust decomposition begins.
Without delving into complex chemical processes, we can say that the method allows you to reverse the oxidative reaction and restore previously damaged areas .
Correctly, this method of fighting rust is not called electrolysis, but the electrogalvanic method. It is used not only in everyday life, for personal needs, but also for a more serious purpose, for example, in the restoration of archaeological finds.
The best corrosion converter sprays
The most popular form of release of corrosion converters is sprays.
WURTH
This German product is made using special chemicals. It is easy and convenient to apply even in hard-to-reach areas. After all, there is a long nose here. This converter has excellent penetrating ability, it is perfectly absorbed and instantly dissolves rust.
WURTH protects against further damage and is suitable for all metal surfaces.
Advantages:
- Excellent penetrating ability.
- Squeezes moisture out of electrical contacts.
- Formation of a protective layer.
Flaws:
- High price.
- Very flammable, you have to be careful.
KUDO
This product is made on an acid basis. KUDO deeply affects rust, removes it and protects it from secondary appearance. After treatment, a film is formed on the surface, which reliably protects the car body from subsequent negative influences. The converter has a lasting effect and is not washed off after treatment.
Advantages:
- Quick and easy application.
- Affordable price.
- Dries quickly.
- No smell.
Flaws:
- There is no embossing effect promised by the manufacturer.
Hi-Gear Rust Treatment No-Rust
This is one of the most famous and popular converters among domestic drivers. This is due to the fact that the product contains quite aggressive components. They quickly eat away rust and turn it into soil. Hi-Gear Rust Treatment No-Rust comes in an aerosol spray form. Powerful directional flow ensures deep and uniform penetration of the transducer.
Advantages:
- Maximum penetration of the product.
- Improves adhesion with various types of varnishes and paints.
- Quick results.
- Long-term protection.
- Comfortable application.
Flaws:
- The product will corrode the paint.
- Can only be used outdoors.
- It is necessary to wash off with alcohol.
Pros and cons of cleaning
Electrolysis, as a method of combating rust, is absolutely safe. The electrolyte solution is not poisonous, but should not be ingested.
The gases released are not toxic . The currents used are of low frequency, so they cannot cause harm to health.
Another advantage of the method is that there is no risk of damaging the part. Even if you keep it in the solution, nothing bad will happen; the self-healing process will not reverse because of this.
In comparison with mechanical and chemical methods of rust removal, electrolysis has one very important advantage. This method does not affect “living metal” , that is, one that has not yet undergone changes.
Abrasives, brushes, acids and other aggressive methods inevitably lead to the fact that some part of the undamaged metal will be removed, but this does not happen during electrolysis.
The disadvantage of this method is that it is not always convenient to use in practice. For example, it may be difficult to clean large parts because it is difficult to find suitable containers for them.
In addition, you will have to spend some time not only on preparatory measures, but also on the cleaning itself.
LiveJournal of Dmitry Babenko
I recently bought a bag of rusty locks on Avito, the locks are excellent, but too rusty. There are several ways to get rid of rust: 1. soak objects in kerosene or diesel fuel - an ineffective method, and also dirty and smelly. 2. polishing the object with a special grinder using a variety of attachments - the method is quite expensive, and the result is a shiny object devoid of the patina of time, and thus - killed, in my opinion. 3. electrolysis - maximum results at minimal costs. What you will need to start an electrolytic workshop at home: 1. DC source, 2. soda ash, 3. piece of stainless steel, 4. 5-liter plastic bottle. First, we take a charger from a mobile phone, its power is quite enough, we cut off its plug - we get two wires, and we go with it to the garages, to the men. Guys in garages enjoy vodka, but they have a device that will show which wiring is “plus” and which, accordingly, is “minus”. We mark one of the postings and remember what we marked. 2. We go to the hardware store and buy a pack of soda ash and a stainless steel spatula. Important: soda ash and baking soda are different substances. We need calcined one. 3. We cut the plastic bottle, now it is a bathtub. 4. Pour water into the bath and add soda at the rate of 2 tablespoons per liter. 5. We screw a steel wire to the “plus” and “minus”, these will be our electrodes; contact of the copper wire with the solution is unacceptable. 6. We screw a spatula to the anode (“plus”), and a lock to the cathode (“minus”) and lower it into the solution. We make sure that they do not touch. 6. Check everything again and plug in the charger. The process begins, small bubbles rise, and the solution becomes dirty before our eyes.
Rules for removing plaque at home
To remove rust from a metal surface by electrolysis, you will need:
a suitable plastic container, such as a bucket or basin;- a steel or stainless steel plate that will act as an electrode - it is better to give preference to stainless steel, since it will last much longer than ordinary metal; it is good if the plate completely surrounds the perimeter of the part being cleaned;
- ordinary tap water;
- soda ash - it is sold in household chemicals departments; housewives use it to wash things;
- battery charger.
To prepare the solution you will need 3 water and 1 teaspoon of soda. The procedure is as follows:
- The prepared solution is poured into the container.
- An electrode is lowered into it.
- Immerse the part that needs cleaning into the solution. This is done in such a way that it does not touch the electrode.
- Connect the power. Polarity must be strictly observed. The electrode must be connected to the positive “+” conductor, and the object to be cleaned to the negative “-”. Contact with parts must be good.
- After completing all preparatory manipulations, turn on the power. If the charger is equipped with an ammeter, you can see how the system begins to pass current.
- After a short time, bubbles will appear on the part. This is completely normal and indicates that the cleaning process has started.
- The duration of the procedure depends on a number of factors. The size of the part and the electrode, as well as the area of rust, matters. Periodically, the system must be turned off, the product removed from the solution and inspected. The average duration of cleaning is 5-6 hours. If the object is covered with a very thick layer of plaque, you can leave it to soak overnight.
- When the cleaning process is completed, the part is removed, washed under running water and inspected. If there are small areas of rust left on the product, they can be cleaned off with a plastic scraper.
At the end of the procedure, the part is dried with a hairdryer or left to dry in the sun. To protect against rust re-formation, you can apply a small layer of lubricant to the metal surface.
What you need to remove rust
You can even use a cut plastic bottle for electrolysis.
How to remove rust with electrolyte? To do this you will need to prepare several items:
- Plastic or rubber container;
- A power source with a voltage of 12-24 V, for example, a computer power supply or a car battery is suitable;
- Wires with stripped ends or terminals;
- A substance that will act as an electrolyte. Any powder that contains sodium will do: baking soda or soda ash, salt, sodium hydroxide;
- Positive anode electrode: a piece of stainless steel or galvanized will do. It is recommended to use soda, as it is the most effective and at the same time safe;
- Water.
Important! Any dielectric container will do.
Helpful information
To ensure that the process of removing rust from metal by electrolysis is as successful as possible, you need to take into account the following tips:
The part should only be processed in a plastic container. Metal buckets or basins are not suitable for this purpose. Their use carries the risk of short circuits or holes.- If there is pitting corrosion on the product, you should not try to remove it by electrolysis. The electrolyte is not able to penetrate the thickness of the metal.
- Once processing is complete, no special disposal measures need to be taken. The solution is simply poured into the sewer; this will not cause any harm to the environment.
Electrolytic plasma polishing
One of the advanced methods of preparing metal products for cars, which allows you to combine several operations in preparation for painting. For example:
- rust removal;
- removal of grease stains (degreasing);
- removal of contaminants;
- surface hardening;
- increased corrosion resistance;
- polishing
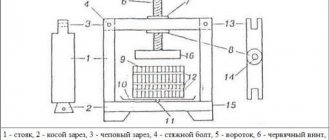
This is especially convenient when cleaning areas with complex shapes (for example, car doors), when other options are difficult or do not give the desired effect. Electrolytic plasma polishing is carried out on a special unit, the schematic diagram of which is shown in Fig. 3.
The anode is the car part being processed, to which “+” is connected from the current source, and the cathode, with “-” connected to it, is a metal bath with electrolyte. It is also possible to use a special plate as a cathode; in this case, the material for the bath is usually plastic.
Consultant for body repair at krasymavto.ru
The technology of such polishing consists in the formation around the treated area - the anode - of a vapor-gas shell with a thickness of 50 to 100 microns, in which the electric field strength reaches 105 V/cm. Such a shell, in fact, is the plasma where processes occur.
Due to this, the electric field strength increases near the microprotrusions, and pulsed discharges occur. Microprotrusions are destroyed - the surface is smoothed. Electrolytic plasma polishing occurs at electrolyte temperatures from 70 to 90 °C, voltage 220-350 V.
Devices for electrolytic plasma polishing are produced by industry. They have different power ratings (from 5 to 1000 kW).
The choice depends on the surface area of the part being processed, in our case, on the size of the car part from which rust needs to be removed. The larger the area, the more powerful the installation should be.
Important! Due to high temperature, high voltage, and high current, it is not recommended to make such units with your own hands. You can purchase them from manufacturers. Only trained specialists should work on such installations.
Neutralization
Neutralization is a necessary stage of the work, since it cancels the effect of the agent that was used to pickle the welds
It is important to neutralize this effect to avoid the possible appearance of efflorescence (salt deposits). This procedure is performed using a unique Inox Fit product, which was developed by Nitty-Gritty
It is applied directly after pickling and polishing of welds, affecting the entire treated surface, while guaranteeing a perfect surface cleanliness. This was made possible due to the higher surface tension compared to water.
Corrosion protection
Follow the simple tips below to protect your car from corrosion:
- wash your car regularly;
- install mudguards (if you don’t have them);
- treat the car with polishing agents (such as “liquid glass”);
- carry out anti-corrosion treatment on the underbody of the car.
Regularly inspect your clean car for problem areas. Remove any small stains that appear in a timely manner. Remember: severe advanced corrosion will lead to expensive body repairs.
Take good care of your car, and then the methods described above will not be useful to you!
Safety precautions
When working with an electrolysis cleaning device, you should follow simple rules that will make the process completely safe.
- Cleaning should take place in a bright room, with the window open.
- Closed clothing with long sleeves and rubber gloves will help protect your body.
- You can’t just plug the device into the network and leave; the process needs to be controlled.
- You should carefully select a power supply. Thus, the recommended operating voltage should not exceed 12 V. Otherwise, there is a high risk of electrocution.
It must be remembered that the work is carried out with current, so it is important to control each stage.
How and with what to remove rust from metal
Removing traces of corrosion from various objects has its own specifics that must be taken into account.
From the body of a car
Rust often forms on the car body. The following products are best suited to remove it:
- Orthophosphoric acid. Its solution is applied to a sponge, which is used to wipe the car body.
- Zinc. Mixtures are made from it that allow you to get rid of contamination after the first treatment.
From a water tap
The optimal remedy for rust on metal, especially for cleaning enameled metal surfaces, is Adrilan, produced for washing household appliances. Since it is very concentrated, it is diluted in warm water before use.
An effective cleaning agent, and not only against rust, but also against any contaminants.
From a bicycle
You can easily get rid of rust stains that appear on a bicycle frame over time using citric acid. Moreover, before removing rust from metal, the surface must be degreased, and then rinsed and dried thoroughly.
From skates
If skates are stored for a long time in conditions of high humidity, a rusty coating begins to form on them. You can remove it using a mixture of baking soda and lemon juice. To do this, the components are mixed until a mushy substance is obtained, which must be applied to the contaminated surface for an hour and a half. After this, the skates need to be washed and dried.
From a horseshoe
An old rusty horseshoe can be cleaned with oxalic acid. To create a solution, you need to mix acid and boiled water in a ratio of 1 to 12. A horseshoe is placed in the resulting liquid for forty minutes, after which it is washed with running water.
From the tools
Metal tools that are rarely used will corrode over time. You can clean them from rusty deposits using a vinegar solution. To prepare it, you need to mix white vinegar with warm water in equal proportions. The resulting liquid is applied to contaminated instruments, after which they can be easily cleaned with a wire brush or sponge.
Once cleaned, polished metal instruments can be treated with a solution of gasoline and wax or paraffin to protect them from further corrosion. To obtain it, you need to add paraffin or wax to gasoline heated in a water bath.
Chrome surfaces
It is impossible to remove rust from chrome surfaces using mechanical methods, as they destroy the coating. The only thing you can use is aluminum foil. You can also clean chrome parts with an acid converter, acetic or citric acid. If the taps in the bathroom or other chrome-plated elements constantly rust, that is, traces of rust appear on them again after 2-4 days, it means that the process has spread to the deeper layers of the material. The best option is to replace the module; anyway, it will not last long.
Knives
Cleaning knives from rust is quite simple, but thin metals and alloys are often used to make them, so there is a possibility of damaging the tool. It is better to choose “soft” cleaning methods. Regular cola containing phosphoric acid will help quickly remove rust from a knife. It is enough to warm the drink to room temperature, and the working composition is ready. Knives damaged by corrosion are soaked in it and left for 30-40 minutes. After this, it is enough to remove the softened plaque with a napkin.
Nuts, chains
You can clean chains and nuts from rust using a vinegar solution. It is enough to dissolve 100 ml of table vinegar (9%) in 1 liter of water and place the rusty elements in the resulting liquid. You need to soak them for about 3 hours, and then just remove the red spots.
Dishes
You can clean rust from cast iron or a regular metal kettle with a metal brush (as soft as possible). If there is any doubt about the quality of the cookware material, it is better to use abrasive substances such as soda and salt
If there is a large layer of rust, it is better to remove it in 2 passes, trying to act carefully. To increase the cleaning efficiency, the metal can be preheated with gas.
Cleaning small household items
All of the above methods can be used to clean small metal objects. The only difference is the ability to place the rusty product entirely into the product being used.
Materials and algorithm for cleaning coins using electrolysis
Cleaning coins with electrolysis is a risky method, especially for a beginner, so you should start with coins that are not particularly valuable. Even ordinary soda can ruin a coin, but electrolysis can cause serious harm to money - completely erase the patina or cause irreparable damage to the coin. It is impossible to get rid of such damage, which means that the value of the coin will decrease.
Cleaning a coin by electrolysis
Cleaning copper coins with citric acid
Citric acid for copper is a highly destructive reagent and you should think very carefully before using it. As a result, you can not only not improve, but also greatly worsen the appearance of the coin and its value. At the same time, citric acid is one of the best options for cleaning silver coins.
| The 1875 penny before cleaning with citric acid looked quite ordinary, but the cleaning process can hardly be called such; the coin was practically destroyed in a few minutes in acid. |
Mechanical cleaning
Mechanical cleaning is a special process for restoring coins in which the coin is not cleaned, but rather cut out or restored in a layer of oxides. For such a reading, the decisive factor is the experience and hard work of the master. The technology can be described very primitively as follows:
- The surface of the coin is desalted and cleaned of dried dirt in distilled water;
- All oxides, including loose ones, are strengthened by impregnating the surface in synthetic resin (paraloid B72);
- Using scrapers, cutters, brushes, and needles of varying hardness, the master removes from the surface everything that he considers unnecessary. By securing unstable areas, they do not fall through and can be aligned with the main field of the coin and no cavities remain. All work is performed under a microscope.
| These 4 kopecks from 1762 were cleaned by a professional using mechanical cleaning technology. The result is simply magnificent, but such processing of the coin took a very long time (2 months for this coin) and is only relevant for rare and valuable coins. |
Rating of liquid products
Among the anti-corrosion liquids for the car body, the following are perfect.
FENOM FN956
This is one of the best liquid corrosion converters. Its effectiveness has been proven by many tests - the FENOM FN956 composition easily transforms rust up to 100 microns thick. After drying, the metal surface is covered with a durable crust based on manganese and iron compounds.
Advantages:
- Rust protection for up to seven years.
- Can be easily used for preserving metal without painting.
Minuses:
- Insignificant volume.
- Great cost.
YasKhim
YasChem is a universal and affordable drug. Essentially, it is phosphoric acid diluted with water and additional components. The drug retains its qualities even after defrosting.
Advantages:
- High efficiency.
- Affordable price.
Minuses:
- There is no effect of tinning, galvanizing or other protection.
Chemist
This product easily removes corrosion and protects the body metal from future relapses. After applying the drug Khimik, a zinc coating appears on the surface and a layer of primer is formed. The latter helps to significantly improve the adhesion of the treated metal to paint and varnish.
Advantages:
- It acts quickly and does not require rinsing.
- Prevents relapse.
- Easily removes rust.
- Reliably protects the surface from corrosion.
Minuses:
- Corrodes paint.
- Toxic.
Brief description of the method
The electrolysis method of cleaning products made of copper, nickel, bronze and other metals has found wide application among treasure hunters and collectors. With some experience and proper application, it allows you to achieve good results.
The complex physical and chemical process can be briefly described as follows:
- the electrodes are placed in a special solution;
- current is passed through them;
- due to electrical influence, certain substances are released at the electrodes;
- an electric field is created.
The method can be used even by those with little knowledge of electricity, but it is important to be careful.