Graphite
- a mineral from the class of native elements, one of the allotropic modifications of carbon. A common mineral in nature. It is usually found in the form of individual flakes, plates and clusters, varying in size and graphite content. There are deposits of crystalline graphite associated with igneous rocks or crystalline shales, and cryptocrystalline graphite formed during the metamorphism of coals.
- Structure
- Properties
- Morphology
- Origin
- Application
- Classification
- Physical properties
- Optical properties
- Crystallographic properties
See also:
Agate
– price and medicinal, magical properties
Physical properties and structure of diamond
Graphite in nature
Found in nature in granites and pyrite.
It is formed in igneous and volcanic rocks, skarns and pegmatites at high temperatures, occurs in quartz veins with various materials, and is widespread in marble, crystalline schists, and gneisses. As a result of pyrolysis under the influence of traps on coal deposits, large deposits of natural mineral are formed. Indicators:
- Mineral content 2.0%
- Carbon content > 98.0%
- Sulfur content 550 ppm
- Temperature range -200…3000°C
- Leachable chloride 50 ppm
- Compressibility 40%
- Regeneration 15%
- pH range 0-14
- Load sag <5%
PHYSICAL PROPERTIES
| Mineral color | iron black fading to steel gray |
| Stroke color | black fading to steel gray |
| Transparency | opaque |
| Shine | semi-metallic |
| Cleavage | very perfect in {0001} |
| Hardness (Mohs scale) | 1-2 |
| Kink | mica-like |
| Strength | flexible |
| Density (measured) | 2.09 – 2.23 g/cm3 |
| Radioactivity (GRapi) | 0 |
Types of natural graphite:
- crucible (used for the production of refractory products. It is characterized by increased thermal conductivity and resistance to sudden temperature changes),
- casting crystal (has a low coefficient of expansion, is characterized by strength at high temperatures, is used when casting parts),
- battery (used as an additive, graphite is used for the production of electrodes, has improved technical and chemical properties),
- for the production of pencil leads (fine, soft, does not contain iron impurities),
- elemental (graphite is used for the production of galvanic cells, it has increased thermal and electrical conductivity),
- electric coal,
- for the production of lubricants and electrically conductive rubber.
Properties of graphite
Pencil lead is made from graphite, which is a form of natural carbon. Under the influence of pressure and heating in the soil, graphite acquires a crystalline structure. Its crystals resemble plates united by a weak bond.
The most common type of graphite is an amorphous, or slate, crystal. It is known that such lead is crumbly and is not suitable for drawing.
About 1,500 years ago, high-density graphite was discovered in England, where it was subsequently mined for three years. Graphite matching the description was used in the manufacture of molds for cannonballs.
High-quality graphite contains a high percentage of carbon. In addition, it turned out that stylus of this property contains the largest proportion of clay in its composition. Thanks to clay, the mixture retains its strong consistency until firing, and after it has a cementing effect.
Crystallographic properties
| Point group | 6mm - dihexagonal-pyramidal |
| Space group | P63mc |
| singonia | hexagonal |
| Cell Options | a = 2.461Å, c = 6.708Å |
| Twinning | by {1121} |
Chemical properties of graphite
Graphite interacts with oxidizing agents and strong reducing agents:
The concept of allotropy
The formula of graphite shows that this substance contains only free carbon. Although in nature it is often found in the form of compounds. Such examples are carbon dioxide and carbon monoxide, limestone, chalk, marble.
The fact is that the formula of graphite in chemistry is the same as that of diamond. Is it possible? It turns out that substances with the same composition have completely different properties. This phenomenon is called allotropy. It can be determined by the number of atoms in a molecule of a substance or their spatial arrangement. An example of the first case is oxygen. If there are two atoms of this chemical element in a molecule, oxygen is formed, and if there are three, ozone is formed.
Graphite: formula, chemical and physical characteristics
As we said, graphite is carbon. Accordingly, in chemistry it is written as C. Despite the fact that the formula of diamond and graphite is similar in qualitative composition, these substances have significant differences in properties. This is explained by the different spatial arrangement of carbon atoms in their molecules.
Graphite is a soft, gray substance with a metallic luster. It effortlessly separates into small plates and conducts electric current. Scientists have proven that if graphite is heated to 1,600 degrees Celsius under a pressure of 104 MPa in the presence of catalysts, it will turn into diamond. In this way, artificial jewelry is produced in industry.
Graphite is not a chemically active substance. It reacts only with certain salts and alkali metals. The products of such reactions are similar inclusions. Combustion of graphite in oxygen occurs only at very high temperatures with the formation of carbon dioxide. However, it undergoes a fluorination reaction. In this case, a white powder is formed, the structure of which changes to a zigzag, acquiring better lubricating properties compared to ordinary graphite.
Structure
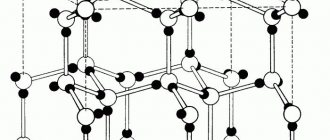
When considering what density graphite has, as well as its properties and types, it is necessary to pay attention to its structure. This is a layered substance. Its carbon atoms are arranged in a crystal lattice, similar to a honeycomb. Hexagons in one layer fit tightly to each other. However, the connection between each level is weak. It is this feature that makes graphite easy to break.
According to the Mohs scale, the hardness of the material is equal to one. For comparison, for diamond this indicator is 10, and for porcelain stoneware – 5. At a temperature of 1500°C, according to scientists’ research, the crystal lattice of graphite can be transformed into diamond.
During industrial processing, the structure of the substance changes. At the same time, different grades of graphite have different properties. If the extracted material has not been processed artificially, it is a natural type of substance.
Optical properties
| Type | single-axis (-) |
| Refractive indices | nω = 1.93-2.07 |
| Anisotropy | excessive |
| Color in reflected light | iron black fading to steel gray |
| Pleochroism | strong, red color |
| Luminescence in ultraviolet radiation | not fluorescent |
PROPERTIES
Conducts electricity well. Unlike diamond, it has low hardness (1 on the Mohs scale). Relatively soft. After exposure to high temperatures it becomes slightly harder and becomes very brittle. Density 2.08-2.23 g/cm³. The color is dark gray, metallic luster. Infusible, stable when heated in the absence of air. Greasy (slippery) to the touch. Natural graphite contains 10-12% admixtures of clays and iron oxides. When rubbed, it separates into separate flakes (this property is used in pencils).
The thermal conductivity of graphite is from 278.4 to 2435 W/(m*K), depending on the grade of graphite, on the direction relative to the basal planes and on temperature.
The electrical conductivity of graphite single crystals is anisotropic, in the direction parallel to the basal plane it is close to the metallic one, in the perpendicular direction it is hundreds of times less. The minimum conductivity value is observed in the range of 300–1300 K, and the position of the minimum shifts to the region of low temperatures for perfect crystalline structures. Recrystallized graphite has the highest electrical conductivity.
The coefficient of thermal expansion of graphite up to 700 K is negative in the direction of the basal planes (graphite contracts when heated), its absolute value decreases with increasing temperature. Above 700 K the coefficient of thermal expansion becomes positive. In the direction perpendicular to the basal planes, the coefficient of thermal expansion is positive, practically independent of temperature and more than 20 times higher than the average absolute value for the basal planes.
Graphite single crystals are diamagnetic; the magnetic susceptibility is insignificant in the basal plane and high in planes orthogonal to the basal planes. The Hall coefficient changes from positive to negative at 2400 K.
Morphology
Well-formed crystals are rare. The crystals are lamellar, scaly, curved, and usually have an imperfect lamellar shape. More often it is represented by leaves without crystallographic outlines and their aggregates. Forms continuous cryptocrystalline, foliated or round radial-radiating aggregates, less often - spherulitic aggregates of a concentric-zonal structure. Coarse-crystalline precipitates often exhibit triangular shading on the (0001) planes.
Crystallographic properties
| Point group | 6 mm - dihexagonal-pyramidal |
| Space group | P63mc |
| singonia | hexagonal |
| Cell Options | a = 2.461Å, c = 6.708Å |
| Twinning | by {1121} |
Place of Birth
The largest deposits of graphite are located in China, Ukraine, Mexico, Canada and South Korea. Mineral deposits are economically beneficial for the country in which it is located. In the process of developing deposits, providing industry with the necessary raw materials. The stone formed clusters of gray color. Graphite ore is mined by open-pit mining, and lump mineral is mined by underground mining.
One of the high-temperature vein mineral deposits is the deposit in Ceylon, which is of great industrial importance. Here graphite veins are located among gneisses. There are similar deposits in Quebec, Montana and England.
Where and how is it mined?
There are commercial-scale graphite deposits on all continents:
- Both Americas - USA, Canada, Brazil;
- Europe – Germany, Greenland, Italy;
- Australia.
The raw materials of each graphite mine can be distinguished by structure, color, and other characteristics.
Russia has three largest deposits:
- Buryatia – high-quality densely crystalline raw materials.
- Krasnodar region (two) – dense, finely crystalline, scaly, graphite shales.
Graphites are formed by coal pyrolysis or under the influence of extremely high temperatures and pressure. For example, the outpouring of magma onto coal deposits.
It is mined by above-ground or underground methods. Graphite crystals are found in shales, marbles, and other organic rocks.
The annual global production of graphite is 600 thousand tons.
Reserves
World reserves of graphite (1978, thousand tons) in capitalist and developing countries: flake - South America, 136; Europe, 3500; Africa, 5442; Asia, 900; densely crystalline - Asia, 2900; cryptocrystalline - North America (without USA), 3084; Europe, 5623; Asia, 6168. For graphite mining, see Art. graphite industry.
Varieties
Natural graphite is diverse, so a classification has been developed according to several criteria.
By composition and scope of application:
- Colloidal. Technical variety, artificial graphite powder. Used by industry.
- Pyrolytic. Artificial material. Found application as the basis of tools for studying microstructures.
- Siliconized. Silicon enriched graphite. Resistant to corrosion.
Natural graphite according to its structure is divided into fibrous, densely crystalline, scaly, and graphite slate. There are also varieties - graphite and graphite mica.
Areas of application
The properties of graphite allow it to be used in everyday life and in industry. Due to its high fire resistance and electrical conductivity, it is used in metallurgy for the production of molds and ladles . Foundries use the powder as a lubricant.
In addition, graphite is added to refractory bricks, grinding and polishing solutions. The most famous use is in the manufacture of drawing pencils. Even the nuclear energy industry uses it at its facilities.
Graphite paint is considered one of the highest quality paints. With the help of such a mixture, it is possible to provide effective protection of cast iron, aluminum, wood and concrete products from corrosion.
In the medical field, graphite is used in the treatment of skin diseases, which are the result of a variety of internal disorders. This element also prevents the formation of scars and adhesions after serious inflammation and has a positive effect on metabolic processes. That is why it is added to many medicines.
How are graphite rods produced?
The most important operation in the production of rods is grinding graphite. The smaller the particles, the smoother the rods will be, the better they will write. Mechanical grinding methods require a long time, and still do not produce sufficiently fine particles. Therefore, mechanochemical methods are used; a surfactant is introduced into a vibration or jet mill, which wets the crushed particles and prevents them from sticking together again. As a result, the sizes of graphite particles are negligible - about one micron.
For clarity, I would like to compare them with something known, but even the finest powder is coarser than pencil graphite. But there is no need to grind the clay, it is already fine enough: its particles have submicroscopic sizes. Graphite is mixed with clay. The hardness of the future rod depends on the ratio in which it is mixed. The hardest will come from pure clay, the softest from pure graphite. Of course, both of these extremes are absurd. It’s just that there is very little graphite in 6T, while there is a lot of it in 6M.
A mixture of graphite and clay is forced through a nozzle and a continuous black snake is automatically cut into individual rods. And then they send it to the oven. Pencil clays are made from kaolinite. At high temperatures, water is released from it and a dense polymer is formed. Therefore, the rod becomes strong, water-resistant and more elastic, its hardness increases by one to one and a half grades.
But due to the evaporation of water, the rod after firing is literally saturated with tiny interconnected pores; the line drawn by it is intermittent and uneven. You have to impregnate the fired rod with waxes - suitable, Japanese, carnauba - or wax-like substances like stearin. By the way, such substances also improve the adhesion of what is written to paper and reduce friction when writing.
Artificial graphite - area of application
Structural, fine-grained, anti-friction and casting graphite is artificially produced. The scope of application of the material is quite wide. Graphite is used for the manufacture of refractory materials, electrical machines and installations, in the chemical, mining and manufacturing industries. It is also used to make pencil leads, paints, coatings and batteries. Graphite is indispensable in the nuclear industry and other highly specialized areas.
Application in the food industry
The presented substance is also widely used in the food industry. To do this, it undergoes certain processing during production. The densities of iron, ethyl alcohol, graphite and sugar are, for obvious reasons, different. But the presented material may both contain and be part of certain substances. It is found in paraffins, ethers, alcohol and even sugar.
You can verify this by conducting a simple experiment. First you need to take a piece of sugar. It is placed on a hard lid and covered with a cap (you can use a thimble). The metal covering the sugar is then heated to high temperatures. Over time, acrid smoke will begin to emerge from under the thimble. If you put a match near it, the gas will burn.
When the smoke stops coming out, you can remove the thimble. A black mass remains on the lid. This is coal. It represents the carbon from which graphite is made.
Where is it used?
Graphite is almost universal. There is nothing unusual in this: the necessary characteristics are laid down at the stage of its processing. So, some require increased thermal conductivity, others require electrical conductivity. Still others are interested in the strength properties of graphite.
Taking into account the conditions of the finished product, the mineral is in demand for the following purposes:
- Production of refractory containers.
- Lubrication for smelting steel and alloys.
- Nuclear reactor rods at nuclear power plants and other units.
Souvenir graphite block - Additive to the composition of plastic products, refractories (ceramics, bricks).
- Source code for parts of electrical appliances, bearings, car springs.
- Paint used in industry and in everyday life as a protective coating against rust.
- Raw materials for the production of artificial diamonds.
- Ingredient of medicines, food paraffins, essential substances, alcohols, sugar.
The most famous use of graphite is in the core of pencils.
Moscow scientists have created a medicine from graphite to treat skin diseases.
Material cost
The sale of graphite and its mixtures is carried out by special organizations that extract and receive the material. Its cost is at a quite acceptable level and depends on the carbon content and dimensions of the crystals. Each grade has its own price - the more carbon in the material, the more expensive it is and the better its technical characteristics.
Graphite is sold both wholesale and retail. It is noteworthy that when purchasing in bulk you can get a very good discount.
The cost of the material also depends on the region of sale. The average price is approximately 45-50 rubles per 1 kg. Graphite products are more expensive.
Ionic type
Oppositely charged ions are located at nodes that create an electromagnetic field that characterizes the physical properties of a substance. These will include: electrical conductivity, refractoriness, density and hardness. Table salt and potassium nitrate are characterized by the presence of an ionic crystal lattice.
Don't miss: the mechanism of metal bond formation, specific examples.
Differences between diamond and graphite
Despite the fact that minerals have similar chemical formulas, they differ sharply from each other both in appearance and from a chemical point of view.
First of all, diamond and graphite have completely different structures from each other. After all, graphite consists of a network of hexagons, while diamond has a cubic crystal structure. The fragility of graphite is due to the fact that the bond between its layers is very easy to break; its atoms easily separate from each other. Because of this, graphite easily absorbs light and is very dark, unlike diamond.
The structure of diamond is different in that one carbon atom is surrounded by four more atoms in the form of a tetrahedral triangle or pyramid. Each atom is the same distance from each other. The bonds between atoms are very strong, which is why diamond is so hard and durable. Another property of diamond is that it can conduct light, unlike graphite.
Is it strange that the formula of graphite is the same as that of diamond, but the minerals are completely different? No! After all, diamond is created by nature under enormous pressure and then very rapid cooling, while graphite is created at low pressure but very high temperature.
STRUCTURE
Hexagonal crystalline polymorphic (allotropic) modification of pure carbon, the most stable under the conditions of the earth's crust. The layers of the crystal lattice can be differently positioned relative to each other, forming a whole range of polytypes, with symmetry from hexagonal system (dihexagonal-dipyramidal type of symmetry) to trigonal (ditrigonal-scalenohedral symmetry). The crystal lattice of graphite is layered. In layers, C atoms are located at the sites of hexagonal cells of the layer. Each C atom is surrounded by three neighboring ones with a distance of 1.42Α
There are two modifications of graphite: α-graphite (hexagonal P63/mmc) and β-graphite (rhombohedral R(-3)m). They differ in the packing of layers. In α-graphite, half of the atoms of each layer are located above and below the centers of the hexagon (laying...AVAVAVA...), and in β-graphite, every fourth layer repeats the first. It is convenient to represent rhombohedral graphite along hexagonal axes to show its layered structure.
β-graphite is not observed in its pure form, since it is a metastable phase. However, in natural graphites the content of the rhombohedral phase can reach 30%. At a temperature of 2500-3300 K, rhombohedral graphite completely transforms into hexagonal graphite.
Making diamond from graphite
The formula of graphite - C - allowed scientists to carry out many experiments, as a result of which allotropic substances of graphite were found.
Teachers tell both schoolchildren and students about how scientists tried to create diamonds from graphite. This story is very interesting and fascinating, and it also allows you to remember the existence of allotropic substances such as graphite and diamond, and their differences.
Some time ago, scientists tried to create diamonds from graphite. They believed that if the formula of diamond and graphite was the same, then they could create a diamond, because the stone was very expensive and rare. Now we know that the diamond mineral appears in nature under high pressure and instantaneous cooling. Therefore, scientists decided to explode graphite, thereby creating the necessary conditions for the formation of diamond. And in fact, a miracle happened: after the explosion, very small diamond crystals formed on the graphite.
Story
The history and time of formation of graphite remains a mystery to science: it is too similar to other minerals in description.
The only clue is clay utensils from the Boyan Maritsa culture (the territory of modern Bulgaria and Romania, 6 thousand years ago). The products are painted with graphite paints.
Abraham Werner suggested calling the mineral graphite. This famous chemist, who “baptized” dozens of stones, took as a basis the property of the mineral to leave a clear trace of color.
The ancient Greek term γράφω means “I write.”
On the territory of Russia, graphite was found in 1826 in the Urals.
In history and literature, the mineral also appears as black/silver lead and iron carbide.
Therapeutic effect
Homeopaths were the first to appreciate graphite. They found that the mineral is suitable for the treatment of skin pathologies (eczema, psoriasis, lichen, etc.).
Today the list has been expanded:
- Metabolic disease.
- Malfunction of the thyroid gland.
- Respiratory tract diseases (rhinitis, bronchial asthma).
- Gastrointestinal problems (gastritis, gastric ulcer, duodenal ulcer, colitis).
- Women's ailments (amenorrhea, chronic inflammation of the ovaries, mastopathy).
- Conjunctivitis, cataracts, stye.
The mineral also “supervises” emotional health. It is prescribed for morning headaches, neurasthenia, apathy, and depression.
Sources
- https://vseprokamni.ru/vidy/organicheskie/ximicheskaya-formula-grafita.html
- https://FB.ru/article/351885/chto-takoe-grifel-iv-ch-m-zaklyuchayutsya-ego-unikalnyie-svoystva-dlya-risovaniya
- https://mineralpro.ru/minerals/graphite/
- https://www.syl.ru/article/307195/formula-grafita-allotropiya-ugleroda
- https://FB.ru/article/277065/grafit-plotnost-svoystva-osobennosti-primeneniya-i-vidyi
- https://vseprokamni.ru/svoistva/kristallicheskaja-reshetka-grafita.html
- https://crystal-wow.ru/kamni/fizicheskie-svojstva-grafita-tablica.html
- https://vseprokamni.ru/svoistva/fizicheskie-i-himicheskie-svojstva-granita.html
- https://natrukodel.ru/prochie/himicheskaya-formula-grafita
- https://abc-24.info/prostoj-karandash-istoriya/
- https://FB.ru/article/236131/allotropnyie-veschestva-almaz-i-grafit-formula-grafita-i-almaza