Some say that after using the converter everything is bad for them. And others say that everything is fine with them.
The trick is that everyone is right. :))
Let's figure out what these people did.
Some applied the converter and waited for it to work. We cleaned off the accumulated deposits with a hard scourer or simply wiped them with a rag, then degreased the part (what matters here!) and primed it.
Others applied the converter and waited for it to work. We washed it off with water (with or without baking soda, it doesn’t matter), degreased it (what matters!) and primed it.
For both the former and the latter, the result can be either positive or negative. :)))
Magic?! NO! Magic doesn't exist.
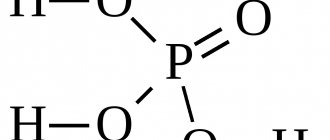
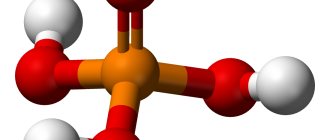
Phosphoric acid (namely, it is the main element in the rust converter) dissolves in water, ethanol and other solvents. For those who don’t know, ethanol is banal alcohol.
From here the following follows. Any rust converter based on this acid can be neutralized either with water or any other solvent. Alcohol is also a solvent if so :))
Further, following common logic, everyone knows that if bare metal is poured with water, it will accelerate the formation of rust almost instantly (and it will not necessarily be visible to the naked eye)
This leads to the following: Wash off the rust breaker from bare body metal with water, idiotic!
And this is where we start to get to the magic.
Why is it that some people washed off the part with water and primed it and everything is fine for them, while others who seem to have done the same thing are doing poorly?
And at the same time, for some who did not wash it off, everything can either be good or bad.
And the magic lies in the pre-priming stage. Namely, degreasing the surface.
———- thinner solvent degreaser——
Before painting, as everyone knows, the surface must be degreased.
And here the dancing with the tambourine begins. Which then can become one of the reasons that will lead to misunderstandings with the rust converter.
The fact is that there are DEGREASERS and there are SOLVENTS. Both are designed to clean the surface.
BUT! SOLVENT AND DEGREASER ARE DIFFERENT PRODUCTS. When painting a car, it is a degreaser and not a solvent that is used to clean the surface before painting.
DEGREASER (SO CALLED ANTISILICONE) ACTS DIFFERENTLY THAN SOLVENT.
The difference between them is the same as between a solvent and a thinner. Car paint cannot be diluted with a solvent; for this purpose, a thinner must be used.
———- thinner solvent degreaser
——
*Thinner and degreaser*
THINNER AND DEGREASER
much
less aggressive
than
solvents
and in addition to this, both THINNER AND DEGREASER, unlike
solvents
, DO NOT LEAVE AN OILY RESTRAINT on the surface after they evaporate. There are nuances in the composition itself, but that doesn’t matter anymore.
Chemical cleaning
Chemical removal of traces of rust is carried out by applying chemical converters. Cleaning metal from corrosion is a labor-intensive process. Khimprom produces three types of chemical cleaning products:
- liquid;
- gel;
- aerosol.
Any rust converter works according to this principle. The main active ingredient of the product is phosphoric acid. After application, the acid actively interacts with the surface of an impermeable film of microscopic thickness. Thus, access of the oxidizing agent to the surface is blocked, the chemical reaction stops and further destruction of the metal does not occur.
Carrying out work requires strict adherence to safety rules and manufacturer’s recommendations for the use of the product.
Chemical categories
Washable - after treatment with them, the metal should be washed, dried well and an anti-corrosion compound applied.
Acid immersion cleansing
If phosphoric acid is available in sufficient quantities, you can get rid of rust by immersing the part in it. Before doing this, you need to completely degrease the damaged item, and then wash off traces of the degreaser. The cleaned part must be neutralized from the action of acids, so it is treated with a solution of alcohol and water with the addition of ammonia.
Then the part must be thoroughly dried. All actions in this process have their own production necessity. If you immerse an unprepared part in phosphoric acid, the etching will be uneven. If the part is not dried, for example by convection, hydroxide will appear on the surface.
Choosing paint
The next question we will face is: what paint to choose? At the moment, the most popular types of paint are:
Oily. These drying oil-based paints are well suited for interior work, but it is better not to use them for exterior work. Oil paint quickly fades, cracks, offers little protection against rust and cannot withstand heating above 80 degrees.
Alkyd. These paints adhere most firmly to the surface, which is why they are often used for galvanized metal. But like the previous ones, oil paints cannot withstand high temperatures and are also flammable.
Acrylic. These paints are suitable for both exterior and interior use. They are durable, environmentally friendly, do not fade and are fireproof. The advantages include resistance to high temperatures, as a result of which the paint can be used for heating radiators.
Epoxy paints can also be distinguished, but they are not used in everyday life because they are very toxic.
If our main goal when painting is to protect the metal from corrosion, it is worth choosing a paint that contains special anti-corrosion additives. Such paints will cost more, but their effectiveness is higher.
Phosphating of metal surfaces
Phosphating is the process of coating the surfaces of ferrous or non-ferrous metals with a thin film that protects it from rust formation and improves adhesion to the paint composition.
The use of this technology can significantly improve the wear resistance of contacting parts in friction units. The method can be implemented for almost all alloys, except for high-alloy steel - a phosphate film of insufficiently high quality appears on it.
Why is phosphating performed?
Phosphating of metal before painting is carried out in order to provide the surface with reliable protection from corrosion processes in places that have been mechanically cleaned of old paint and rust. Before applying a protective layer, metal structures or products must be thoroughly cleaned of dust and dirt, and also degreased.
This method of protecting metal structures allows their operation in the following conditions:
- exposure to automobile oils and fuels;
- in electrical installations up to 1 kV;
- high humidity;
- in environments with organic solvents;
- being under paintwork.
The resulting film is capable of reliably protecting the metal under the above conditions, but is quickly destroyed in aggressive acidic and alkaline environments. Therefore, before performing phosphating, it is necessary to determine the composition of the environment in which the metal product will be used.
Step-by-step instructions for making a rust converter with your own hands
Products called “Rust Converter” help solve the problem of corrosion of metal products.
Today there is a wide range of such chemicals on sale. However, the issue of making anti-rust products on their own is of interest to many.
Having decided to use a homemade converter, you must follow the recipe and technology for its use. At the same time, it is worth remembering all the pros and cons of such a product, as well as precautions when using it.
We'll tell you how to make a rust converter with your own hands at home in this article.
Pros and cons of cleaning
Phosphoric acid is a popular and sought-after rust remover.
This is due to several advantages of the reagent:
- high cleaning efficiency;
- ease of use;
- budget cost;
- wide scope of application.
However, when using this inorganic substance, a number of negative aspects are revealed. The existing disadvantages limit the use of the converter and force one to remember precautions.
The most important disadvantages are:
- Acid and its vapors can irritate mucous membranes, respiratory tract, and eyes.
- If a pure substance accidentally enters the stomach, nausea, vomiting, and diarrhea occur.
- Possible appearance of holes when cleaning very thin products from rust.
- Cannot be used to remove rust from objects coated with acrylic.
Phosphating methods
The formation of a phosphate protective film on a metal surface is achieved in several ways, the possibility and feasibility of which depends on the size of the structure and its area of application.
The most commonly used methods are:
- surface treatment with Majef, which is allowed even for low-carbon steel, resulting in the formation of a high-quality primer with anti-corrosion properties;
- the use of phosphoric acid or “cold phosphating”, in which the thickness of the protection is no more than 5 microns;
- the use of zinc monophosphate, which is used primarily in the mechanical engineering and electrical power industries;
- treatment with phosphating paste.
To prepare metal for painting, it is necessary to perform a number of mandatory procedures, without which high-quality painting and, accordingly, long-term operation of metal structures are impossible.
You may also be interested in knowing which metal fence paint is best for your situation. Read about this in the article about painting metal fences.
Expert advice
You need to clean areas of rust very carefully so that no holes appear and the metal does not become too thin. It is not recommended to use discs with very large abrasive particles during work, as instead of removing rust, they can cause more harm than good.
Before starting all repair work to remove rust, you must remember that this product is very powerful and strong. After all, it can remove not only the rust itself, but also the decorative coating: chrome, zinc. Therefore, it is necessary to process car parts very carefully and carefully so that you do not have to spend money on new spare parts in the future.
If all work to remove rust using phosphoric acid was carried out in accordance with all standards, then the resulting surface will be durable and reliable. And although the use of converters is considered the most popular method for body work, we should not forget about other options known to craftsmen.
A little converter is applied to a surface cleaned of a thick layer of rust and left for a couple of minutes or hours, depending on the concentration of the solution, and then further repair work begins.
Previous MaterialsUsing camouflage vinyl film for a car Next MaterialsThe best anti-corrosion agent for a car today: rating of anti-corrosion body treatment products
Metal degreasing
Degreasing of structures is carried out to ensure good adhesion of the metal with the paint and varnish composition and primer.
To degrease metal before painting, in principle, you can use any composition that removes organic substances and fats. But still, it is better to use complex compounds that convert rust into a useful layer and prevent its appearance in the future:
- White Spirit;
- numbered nitro solvents;
- degreaser with complex alcohols;
- kerosene.
It is not recommended to use gasoline as a degreasing agent, since as a result of its exposure, an invisible oil film appears on the surface, which impairs adhesion to the paint.
Degreasing must be carried out in well-ventilated areas with constant air circulation, since the vapors of most chemicals used are very toxic. To avoid poisoning, it is recommended to wear a respirator, work in rubber gloves and safety glasses - if any solvent gets into the eyes, a chemical burn to the mucous membrane cannot be avoided.
How to remove rust?
Removing rust with phosphoric acid
Simply painting over the rust will not work. Or rather, it will work out, only the iron oxide remaining under the paint, like a virus, will infect another, “healthy” metal. Therefore, the first stage of restoring a car’s paintwork is removing existing rust. There are several ways.
In this article we will pay attention to the chemical, which is based on the use of phosphoric acid. Its use against rust is widespread.
Article on the topic: Replacing the front and rear crankshaft oil seals with your own hands
Where to buy phosphoric acid?
In auto chemical goods, electronics, radio equipment stores, you can find it at construction markets, and, of course, order it on the Internet. Phosphoric acid is sold where other acids, paints, solders, and various reagents are also sold. It is rarely found in its pure form; more often it can be found as part of a rust converter based on orthophosphoric acid (almost all).
What is orthophosphoric acid?
This is an inorganic compound that is highly soluble in water. It is used in the food industry (as part of chemical phosphate fertilizers), in medicine (dentistry), in the production of household chemicals (as the main component for rust converters and primers for metal).
Safety rules when working with acid
The product belongs to the category of hazardous substances, which affects not only the methods of transportation and storage, but also requires compliance with safety standards during operation. Since it is an acid, it can corrode many materials and cause burns on the skin. Therefore, working with phosphoric acid
This can only be done in special clothing that will protect the skin from contact with hazardous substances.
Don't forget about vapors, which are also very dangerous. When they enter the body through inhalation, they cause serious burns to the respiratory tract. It is very important for every person dealing with such substances to know the procedure to follow if they come into contact with the skin.
- First of all, you should immediately remove all clothing if a harmful substance comes into contact with it.
- Rinse the affected area of skin with plenty of water, preferably running water, for at least 15 minutes.
- Do not rub this product into the skin or wipe it with napkins.
- If rinsing with water does not help, then it should be extended for another 15 minutes. The same action must be repeated when the burning resumes some time after rinsing.
- In order not to aggravate the situation, before the ambulance arrives, it is necessary to protect the affected area from the environment by applying a loose gauze bandage.
- You can take a painkiller. The optimal drug is Analgin.
To ensure that the use of orthophosphoric acid is safe and that you do not have to seek help from doctors, all work should be carried out with special care and precision. If an accident does occur, you should not leave things to chance, since any chemical burns can be extremely dangerous.
Rules for transporting acid
Acid can be transported with strict adherence to the following precautions:
- the packaging must be chemically inert (preferably plastic - glass cannot ensure safety during transportation due to its fragility);
- the container must prevent spilling or evaporation of the liquid preparation or scattering of crystals;
- It is necessary to exclude the possibility of foreign moisture or dust getting inside the package.
Important! Safety signs must be placed on the outside of the container.