Since ancient times, copper objects have been in demand among people. Currently, the material is valued for its decorative properties. However, melting copper at home is not easy. Craftsmen try different methods to carry out this procedure on their own.
Copper can be melted at home
Being in nature
The metal received its Latin name Cuprum from the name of the island of Cyprus, where they learned to mine it in the third millennium BC. e. In the periodic system, Cu received number 29, and is located in the 11th group of the fourth period.
In the earth's crust, the element is in 23rd place in distribution and is most often found in the form of sulfide ores. The most common are copper luster and pyrite. Today, copper is extracted from ore in several ways, but any technology requires a step-by-step approach to achieve results.
- At the dawn of the development of civilization, people were already obtaining and using copper and its alloys.
- At that time, it was not sulfide ore that was mined, but malachite ore, which did not require pre-roasting.
- A mixture of ore and coals was placed in a clay vessel, which was lowered into a small pit.
- The mixture was ignited, and carbon monoxide helped the malachite to be restored to the state of free Cu.
- There is native copper in nature, and the richest deposits are in Chile.
- Copper sulfides often form in medium-temperature geothermal veins.
- Often the deposits are in the form of sedimentary rocks.
- Copper sandstones and shales are found in Kazakhstan and the Chita region.
Colors of tarnishing of metals, determination of temperature by the color of a heated workpiece
Metal tarnish colors are a spectrum of colors that form on the surface of a metal when an oxide film appears. These oxide films are created from the metal itself when heated. An important condition for the formation of such a film is the absence of exposure to water.
Such discoloration of the metal is a defect in the welded joint.
Origin of metal tarnish colors
In nature, tarnish colors can be observed on the surfaces of a number of minerals, among them pyrite and chalcopyrite.
It is logical to conclude that these changes are visible as a result of oxidation of the upper layer of the material. As a result, they are covered with a thin oxide film, which refracts the light falling on its surface.
The resulting interference effect “colors” the metal surface in different colors.
The brightness of the tarnish colors depends on the thickness of the oxide film formed and the wavelength of light that hits the surface of the material. The brightest shades can be seen on copper minerals. The resulting colors also depend on the composition of the metal. If an element contains many metal ions, it turns blue. If chromophores are present, you will see red colors.
The artificial color of tarnishing a metal appears on its surface when exposed to high temperatures. An important condition is the absence of water and any other liquids.
As it heats up, the resulting oxide film decreases, which is explained by diffusion (the process of “mixing” or penetration of particles of a chemical element into another material). Specifically, in the situation with a metal oxide film, interaction between oxygen and metal atoms is observed.
It is worth noting that on alloy steels the tarnish color will appear with greater heat than on carbon steel.
Creating artificial tarnish flowers
In the field of metal processing, bluing is actively used. At the same time, the technology of coating alloys with oxide films has been known and has been actively used for thousands of years.
Blued metal is resistant to rust, more durable against mechanical stress and has a beautiful color even without additional coatings and paints.
Burnishing is performed as follows:
- The workpiece is dipped or wiped with mineral oil;
- Heat on a metal sheet to the appropriate temperature (it may differ for different metals and alloys);
- Afterwards they can perform hardening in cold oil (to avoid “metal tempering”).
The resulting oxide layer on the surface of the metal product is completely resistant to water and also has high strength to mechanical stress.
Tables 1.
Oxide films form at different rates and are influenced by the following factors:
- Hardening of the part (the presence of hardening accelerates the appearance of tarnish);
- Presence of contaminants (when heated, contaminants become charred and complicate the formation of a uniform layer of oxide film);
- Roughness. A workpiece that has unevenness receives a dense film and, as a result, the beautiful iridescence of colors may not be visible. A polished part quickly forms a uniform thin layer of oxides on the surfaces;
- Heating technologies. Depending on the equipment used to heat the parts, oxide films are formed at different speeds and different thicknesses. To heat parts, it is best to use equipment that allows you to control and maintain the desired temperature stably.
Thin oxide films absorb light waves with a shorter wavelength, but reflect them with a longer wavelength. The color of metal parts changes depending on the temperature and density of the oxide film. The thicker the oxide film, the lighter the color. Blue or violet color is produced when the longest wavelengths are reflected from the spectrum.
If the oxide film reflects short wavelength waves, the metal surface turns yellow. Light colors correspond to a high heating temperature, light colors correspond to a lower heating temperature.
For this reason, many craftsmen often use tarnish colors to determine the degree of hardening of products, steel filings and cutting tools used during turning operations.
Despite these factors, it is impossible to accurately determine the temperature of the metal using tarnish colors, because the value of this indicator is influenced by the following factors:
- heating time: the period of time during which a metal part heats up to ambient temperature in the absence of heat transfer.
- the presence of various impurities in the metal composition;
- features of lighting in the room where welding or hardening of workpieces was carried out;
- heating rate: change in the temperature of a product per unit time when it is heated.
Among modern devices, there are pyrometers that provide fairly accurate temperature control. They work based on the analysis of laser beams. The devices are equipped with special sensors that analyze reflected laser beams and display the temperature of the metal, which corresponds to the obtained radiation characteristics.
Technologies using tarnish colors are actively used in the production of working tools and equipment. The use of this technique is especially common when working with copper, iron, aluminum and brass.
Hardening improves the following parameters of the metal surface:
The color of the tarnish of the metal and its temperature or the temperature of the colors of the tarnish of the metal
As has already become clear from the material described above, the temperature and color of the metal changes all the time the workpiece is heated.
It is important to note that the tempering temperature of a metal differs for each individual alloy and type of metal.
Therefore, there are a large number of tables and lists of color and temperature relationships. Below are tables of metal tarnish color for different alloys.
Various metals and alloys are used for thermocouples.
So, for example, to measure temperatures 1000–1300°
The thermocouple is made
of platinum and an alloy of platinum and rhodium.
For temperatures 700–950°
a thermocouple is used -
chromel (chromium-nickel alloy) and alumel (aluminum-nickel alloy);
at even lower temperatures, iron-constantan (copper-nickel alloy) and copper-constantan thermocouples are used.
Temperature of hot metal
can be determined with an optical pyrometer - by comparing the brightness of its glow with the filament of an electric light bulb.
In Fig. 63, a
an optical pyrometer
is shown . The pyrometer lens is pointed at a hot object. A light bulb glows inside the pyrometer. In the field of view of the eyepiece, both the filament and the red-hot metal are visible.
Changing with a rheostat
The strength of the electric direct current feeding the electric lamp is selected such that the brightness of the filament of the electric lamp and the hot metal coincide (Fig. 63, b).
Depending on the magnitude of the current, the needle of the device will deviate on the scale at a different angle. For convenience, the scale is graduated in degrees Celsius.
Approximate methods for determining the metal temperature value
In addition to the listed methods, in the practice of heat treatment, approximate methods are used that give only approximate values of the metal temperature.
These methods include determining the temperature of the metal by heat colors
when heated for hardening or annealing and
determining the temperature of the metal during tempering by tarnish colors
appearing on the light surface of the parts (Fig. 64).
Colorful temperature
The main sources of light in nature are heated bodies. For a completely black body, the spectrum of visible radiation, which depends on the heating temperature, measured in Kelvin (K), is called the color temperature (Fig. 1).
An absolutely black body is a physically idealized object that absorbs all radiation, reflects nothing, but can still emit its own radiation.
Rice.
1 - Black body radiation
A similar effect can be observed when heating a metal, which at different temperatures has a different glow color. At first it will be dark red, then red, then orange, then white. So the blacksmith can visually bring the heating of a certain metal to the required temperature (Fig. 2).
Rice. 2 - Glow of heated metal
The operating principle of an electric incandescent lamp is based on the use of this property: an electric current is passed through a thin tungsten wire, as a result of which it heats up and emits radiation in the visible spectrum.
Moreover, the color of the glow can be quite accurately estimated depending on the heating temperature: ~ 600 K - dark red, 1000 K - orange, 2000 K - yellow. The radiation from the surface of the Sun, caused by thermonuclear reactions, has a temperature of about 6500 K, which is already perceived by us as white.
The star Vega has a color temperature of 8000 K to 1000 K and is perceived as white-blue (Fig. 3).
Rice.
3 - Color temperature of a completely black body
Since for different bodies, depending on the chemical composition and physical properties, heating to a given temperature gives a slightly different emission spectrum or may differ altogether (for example, fluorescent lamps), a correlated color temperature is used. It corresponds to the color temperature of a completely black body, similar to the color of the light source in question. In this case, the composition of the radiation and the physical temperature, as a rule, differ.
Physical properties
The metal is ductile and in the open air it becomes covered with an oxide film in a short time. Thanks to this film, copper has its yellowish-red tint; in the lumen of the film, the color can be greenish-blue. In terms of thermal and electrical conductivity, Cuprum is in second place after silver.
- Density - 8.94×103 kg/m3.
- Specific heat capacity at T=20 ° C - 390 J/kg x K.
- Electrical specific at 20−100 ° C - 1.78×10−8 Ohm/m.
- Boiling point - 2595 ° C.
- Specific electrical conductivity at 20 ° C is 55.5−58 MS/m.
Effect of temperature - The phenomenon of brittleness in steels - Material and its work in construction
Heat treatment of steel parts is carried out in cases where it is necessary either to increase the strength, hardness, wear resistance or elasticity of a part or tool, or, conversely, to make the metal softer and easier to machine.
Depending on the heating temperature and the method of subsequent cooling, the following types of heat treatment are distinguished: hardening, tempering and annealing.
In amateur practice, you can use the table below to determine the temperature of a hot part by color.
| Heat color of steel | Heating temperature, °C |
| Dark brown (visible in the dark) Brown red Dark red Dark cherry red Cherry red Light cherry red Light red Orange Dark yellow Light yellow Bright yellow | 530-580 580-650 650-730 730-770 770-800 800-830 830-900 900-1050 1050-1150 1150-1250 1250-1350 |
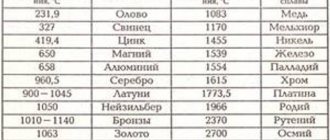
At what temperature does copper melt?
Melting occurs when a metal changes from a solid state to a liquid state. Each element has its own melting point. Much depends on the impurities in the metal . The normal melting point of copper is 1083 ° C. When tin is added, the temperature drops to 930-1140 ° C. The melting point here depends on the tin content of the alloy. In an alloy of cuprum and zinc, melting occurs at 900-1050 ° C.
When any metal is heated, its crystal lattice is destroyed. As it heats up, the melting point increases, but then levels off once a certain temperature limit is reached. At this moment the metal melts. It melts completely and the temperature rises again.
When the metal cools, the temperature decreases, at a certain point it remains at the same level until the metal hardens completely. After complete hardening, the temperature drops again. This is demonstrated by the phase diagram, which shows the temperature process from the beginning of melting to solidification. When heated, heated copper at 2560 ° C begins to boil. Boiling is similar to the boiling of liquid substances, when gas is released and bubbles appear on the surface. At the moment of boiling at the highest possible temperatures, the release of carbon formed during oxidation begins.
Metal tarnish colors
Tarnish colors are a spectrum of colors formed on the surface of iron alloys as a result of the appearance of an oxide film. They are formed when metal surfaces are heated to certain temperatures without the participation of water. Tarnished colors are a defect in the welded joint.
Origin
In nature, tarnish colors form on the surface of many minerals, including pyrite and chalcopyrite. Due to oxidation, they become covered with a thin oxide film, which refracts sunlight. As a result of interference, the metal surface is painted in different colors.
The brightness of the tarnish depends on the thickness of the oxide film and the wavelength. The brightest tarnish colors form on copper minerals. Also, the color depends on the quality of the metal. If an element contains a large number of metal ions, it turns blue.
In the presence of chromophores, minerals turn red.
Tarnish colors can also form naturally on the surfaces of old glass or coins. Discoloration may be due to prolonged contact of these materials with the ground.
If there is a fatty film on them, they turn rainbow-colored. The tarnish hides the real color of the metal. Therefore, it is impossible to determine its true color on a fresh fracture.
It is recommended to determine the color by examining the oxide film.
Artificial tarnish colors are formed on the surface of metal workpieces during welding or hardening. They appear when metals are heated to critical temperatures without the participation of water molecules or other liquids. During heating, the formation of an oxide film occurs.
Its thickness is several molecules and decreases as it heats up. This is due to the phenomenon of diffusion - the process of penetration of the smallest particles of one chemical element into another. In this case, the interaction of metal and oxygen atoms occurs.
On carbon steels, oxide films appear faster than on alloy steels.
The process of coating steel and iron with a layer of oxide film is called bluing. After this procedure, the corrosion resistance of the product increases. Treated parts do not rust. The bluing procedure allows you to give the product a color, even if the metal surface, due to operating conditions, cannot be painted.
During bluing, the workpiece is rubbed with mineral oil and heated on an iron sheet. After the oil liquid burns out, tarnished colors appear on the workpiece. For the desired color, it is necessary to heat the part to the appropriate temperature. The resulting oxide layer is moisture resistant and is not exposed to air.
The rate of formation of oxide films is influenced by the following factors:
- Surface structure: hardened parts oxidize at a faster rate.
- Contamination of the product: surfaces coated with oil become charred during prolonged heating, which leads to the formation of soot. For this reason, an uneven and thin oxide film is formed.
- Presence of roughness: if a workpiece with a rough surface is heated, the oxide film becomes dense. If a part is polished before the heat treatment procedure, a thin film of oxides is formed.
- Heating equipment: If special heating furnaces are used during heat treatment, capable of maintaining a stable temperature, then the oxide film will be dense. At home, you can also use ovens, gas burners or metallurgical furnaces (forges).
Thin oxide films absorb light waves with a shorter wavelength, but reflect them with a longer wavelength. The color of metal parts changes depending on the temperature and density of the oxide film. The thicker the oxide film, the lighter the color. Blue or violet color is produced when the longest wavelengths are reflected from the spectrum.
If the oxide film reflects short wavelength waves, the metal surface turns yellow. Light colors correspond to a high heating temperature, light colors correspond to a lower heating temperature.
For this reason, many craftsmen often use tarnish colors to determine the degree of hardening of products, steel filings and cutting tools used during turning operations.
Despite these factors, it is impossible to accurately determine the temperature of the metal using tarnish colors, because the value of this indicator is influenced by the following factors:
- heating time: the period of time during which a metal part heats up to ambient temperature in the absence of heat transfer.
- the presence of various impurities in the metal composition;
- features of lighting in the room where welding or hardening of workpieces was carried out;
- heating rate: change in the temperature of a product per unit time when it is heated.
In modern industry, temperature control is carried out using special devices - pyrometers. They are equipped with special sensors that determine the degree of heating of the workpiece using a laser.
Tarnish colors are used in the manufacture of working tools, laser marking and external processing of iron, copper, aluminum and brass products.
If it is necessary to produce tools with a high density (razor blades, objects for surgical operations, cutting edges of incisors and grabbers), then the tarnish should be of a bright color: red, orange or yellow.
Tools used in the woodworking sector are heated to purple and green tones. To achieve elasticity when making saws, knives, forks and springs, it is necessary to heat the workpieces until blue or black colors appear.
During the heating process, the metal workpiece becomes flexible, which allows the master to give it the desired shape. After this process, the product is hardened at certain temperatures.
According to the recommendations of experts, the optimal temperature for hardening metals is 700–800 °C. In this case, the product is painted in different shades of red or pink. If these values are exceeded by 300 °C, the workpiece turns orange or yellow.
At high temperatures, overheating occurs, which negatively affects the strength of the product.
Hardening improves the following parameters of the metal surface:
- Hardness: This indicator is nominal. It is written in the Rockwell scale and measured in HRC. Hardness determines the degree of resistance of a metal to mechanical damage. When soft products come into long-term contact with other surfaces, marks remain, which impairs their cutting properties. The hardness of European knives is 60 HRC, Asian knives are 70 HRC.
- Elasticity: this parameter determines the degree of deformation of the metal during bending and impact. If the steel is hardened, when bent by 10-30° it will return to its original position. When overheated, the elasticity of the surface decreases, which leads to tool breakage.
- Wear resistance: this criterion shows the overall resistance of the metal (resistance to abrasive wear, resistance to heavy loads). With proper hardening, the product will be able to function stably for a longer period.
After hardening, the workpiece acquires high hardness. To restore its strength, it is necessary to carry out a tempering procedure, which is repeated heat treatment of the part.
The metal product is heated to lower temperatures and cooled. Between hardening and cooling, the metal surface is also completely cooled by immersing it in a salt solution or oil.
When choosing a vacation, you must consider the following features:
- For products subject to deformation or shock loads, high-temperature tempering must be used: up to 700 °C.
- For light blades, medium temperature tempering is used: up to 500 °C.
- To ensure optimal hardness, low-temperature tempering is used: up to 250 °C. But in this case, the product will not be able to withstand high impact loads and will be easily deformed.
Temperature of colors of tarnish and heat
During the holidays, incandescent colors appear. From them you can determine to what temperature the workpiece has heated up. Unlike tarnish, heat colors change as the metal surface cools. The transition between colors is carried out in a strict sequence, but at a fast speed, so the master must carefully control the heat treatment process.
Steel tarnish color scale
The color of carbon parts at appropriate temperatures is indicated in the following steel tarnish color scale:
Temperature of tarnish colors for carbon steels
| Color | Temperature limits, °C |
| Citric | 220 – 229 |
| Yellow (straw color) | 230 – 245 |
| Gold | 246 – 255 |
| Earthy or brown | 256 – 264 |
| Scarlet or red-orange | 265 — 274 |
| Purple | 275 – 279 |
| Amethyst | 280 – 289 |
| Heavenly | 290 – 294 |
| 295 – 299 | |
| Indigo Crayola | 300 – 309 |
| Light blue | 310 – 329 |
| Aquamarine | 320 — 339 |
On workpieces made of stainless steel 12Х18Н10Т, containing 18% chromium, 10% nickel and 1% titanium (values defined in GOST 5632-2014), tarnish colors are formed at other temperatures.
This is due to the fact that this material is corrosion-resistant and heat-resistant.
Therefore, during quenching and cooling, the smallest particles of metals and oxygen interact more slowly, which prevents the formation of an oxide film during quenching and heating.
The following table of tarnish colors shows the characteristics of color changes in stainless steel products:
Temperature of tarnish colors for stainless steels
| Color | Temperature limits, °C |
| Light straw | 300 – 399 |
| Golden | 400 – 499 |
| Earthy or brown | 500 – 599 |
| Red or purple | 600 – 699 |
| Blue or black | 700 – 779 |
Rainbow streaks may appear on the surfaces of stainless steel workpieces. They may appear when the product is heated to boiling temperature (100 °C). The appearance of rainbow marks is due to changes in the crystal lattice of the metal. The rainbow color on the surface of the workpiece does not indicate overheating of the stainless steel.
Cuprum: element characteristics
The scientific name of copper Cuprum comes from the name of the Greek island of Cyprus, where copper mining began in the middle of the third millennium BC. In the periodic table of Mendeleev, the chemical element copper has atomic number 29 and is located in group 11 of the fourth period. Belongs to the ductile transition metals. In its pure form it has a characteristic golden-pink color. Pure copper is easy to oxidize, so under natural conditions it always forms a thin oxide film on its surface, which gives it a reddish tint.
Physical properties
This is the second metal after silver in terms of electrical conductivity, which makes it extremely popular in modern electronics. The second valuable quality is high thermal conductivity, which allows it to be widely used in all kinds of heat exchangers and refrigeration equipment.
- Melting point 1083 degrees.
- Boiling point 2567 degrees.
- The resistivity at 20 degrees is 1.68·10 -3 Ohm·m.
- Density 8.92 g/cm.
Being in nature
It occurs in nature in native form and in the form of compounds.
The largest deposits of native copper are located in the United States in the Lake Superior region. It was in this area that the largest copper nugget weighing 3560 kilograms was found. There is also a lot of native copper found in the ore mountains of Germany.
In Russia and the post-Soviet space, copper is mined by extracting it from sulfide ore. It can be mined by extracting it from copper pyrite or chalcopyrite CuFeS2. The most famous deposits are Udokan in Transbaikalia and Dzhezkazgan in Kazakhstan.
Copper sulfites most often form in so-called mid-temperature hydrothermal veins. They can also form in sedimentary rocks in the form of cuprous sandstones and shales.
As a rule, copper ore is always mined by open pit mining. The percentage of pure copper in the ore ranges from 0.2 to 1.0 percent depending on the deposit.
Copper alloys
They are the very first metal alloys, the production of which humanity mastered at the very dawn of its development. At what temperature copper melts depends on what alloy it is in. Currently, the most famous and in demand alloys are:
- Brass. An alloy with the addition of zinc, the content of which can reach up to 40%. Zinc increases the ductility and strength of the metal. The temperature at which brass melts is 880 - 950 degrees.
- Bronze. An alloy with tin, with the addition of some other components such as silicon, beryllium, lead. Man learned to make bronze from copper at the very beginning of the Bronze Age. Bronze did not lose its relevance even with the advent of the Iron Age; for example, at the beginning of the 20th century, gun barrels were made from so-called gun bronze. The temperature at which bronze begins to melt is 930 - 1140 degrees.
- Cupronickel. In addition to copper, it contains 5-30% nickel. Nickel increases the strength of the copper alloy and increases its electrical resistance. In addition, corrosion resistance is greatly improved. Melting point - 1170 degrees. In its external characteristics, cupronickel is very similar to silver; it used to be called white copper. But it has higher mechanical strength than ordinary silver.
- Dural, or duralumin. The bulk of the alloy is aluminum 93%, copper accounts for 5%, the remaining 2% is occupied by manganese, iron and magnesium. The name comes from the name of the German city of Düren, where this high-strength aluminum alloy was first produced in 1906. One of its features is the fact that its strength characteristics tend to increase over time. Therefore, it does not lose its strength after several years of use, like other metals. Currently, this alloy is the basis of aircraft construction.
- Jewelry alloys. Alloys of copper and gold. This increases the resistance of the precious metal to mechanical stress and abrasion.
Read also: Can a burner be used as a soldering iron?
Tuesday, November 1, 2016
Draw a graph of copper melting
Draw a graph for the melting of copper. Plot the temperature vertically (1 cell 20 degrees Celsius), and time horizontally (1 cell 10 minutes). The initial temperature of copper is 1000 degrees, the heating time to the melting temperature is 20 minutes, the time for copper to transition to the liquid state is 30 minutes.
The melting point of copper is 1085 degrees Celsius. First, within 20 minutes, the temperature of the copper will increase from 1000 degrees to 1085 degrees, then the temperature will remain constant until the end of melting, then it will increase again. To determine the rate of temperature increase during heating, it is necessary to divide the difference between the melting temperature and the initial temperature by the time of heating to the melting temperature. Thus, the first section of the graph will be a graph line T = 1000 + 4.25t, where T is temperature, t is time.