One of the methods of producing a convex or depressed pattern on metal products, which appeared relatively recently, is called etching. The operating principle of this method is based on the use of electrochemical processes in a liquid electrolyte. If you have artistic abilities, even at home you can get a high-quality pattern with a minimum of required materials and equipment.
When doing etching yourself at home, you will need the following consumables and equipment:
- a product intended for decoration - various cutlery, hunting or camping knives, or simple soap dishes on which simple patterns can be made;
- a container of sufficient volume and convenient shape made of non-metallic materials, suitable for placing the entire product being processed or its part to be decorated. It is most convenient to use glass containers for this purpose, which allow visual control of the processing process.
- a sufficient amount of ordinary table salt;
- nail polish of any color;
- nail polish remover intended for cleaning the treated product;
- source of direct electric current of low voltage. In this capacity, a charger can be used to charge car or telephone batteries.
Drawing
We start by preparing the product to be processed. It must be thoroughly cleaned of grease and dirt, rinsed with hot water and dried. After this, use a brush to apply varnish to the surface of the object to be treated (in our case, these are tablespoons).
Without allowing the coating to dry completely, apply a pattern or inscription.
Depending on the design option and the desired decorative effect, when etching metals, a pattern can be obtained either pressed into the material of the product or protruding on its surface. This can be achieved by removing the protective varnish layer. It is pure metal without a beautiful film that will be subject to chemical etching.
If you want to get a convex image, leave the varnish on it and remove it from the background.
Otherwise, remove the varnish film exactly in the form of the desired pattern - it will be “pressed” into the material of the product. To obtain a fine pattern, it is convenient to remove varnish from the surface with a sharpened wooden stick or toothpick. Try to get the smoothest possible lines without smudges, which can significantly spoil the entire work of etching the design on the product.
| Technology and crafts |
| Technologies for working with metal |
| Chemoplasty |
Chemoplasty
Chemical solutions for pickling iron and steel
The simplest effective solutions for etching iron and steel parts are dilute inorganic acids, especially 20% sulfuric acid, in which etching is carried out at 45-50 ° C, or 20-25% hydrochloric acid, in which parts are etched at room temperature. For etching, 10-15% phosphoric acid is also used, heated to 60-70°C. Parts are etched in it, which will then be varnished or their surface will be left without further processing. If, after etching, galvanic coating of the surface is provided, then this bath is unsuitable.
Chemical etching of non-ferrous metal surfaces
Etching copper and brass
On brass the solution forms a light yellow coating, on copper it forms a light pink coating. The solution contains:
Concentrated nitric acid 250 ml Concentrated hydrochloric acid 150 ml Denatured ethyl alcohol 100 ml Water 500 ml
The parts are etched by briefly immersing them in a bath of solution, after which they are removed and immediately washed with water.
Brushed copper etching
After etching on copper, you will get a rough (to matte) surface. Bath composition:
Nitric acid 40% 600 g Concentrated sulfuric acid 400 g Sodium chloride 3 g Zinc sulfate 2 g
Brilliant etching of copper and its alloys
Concentrated sulfuric acid 500 ml Concentrated nitric acid 500 ml Concentrated hydrochloric acid 10 ml Carbon black 5 g
The operating temperature of the bath is 18-20°C. The degreased parts are immersed in a bath of solution for 10-30 seconds, after which they are removed, washed with water and dried.
Solution for etching aluminum and its alloys
The aqueous solution contains:
Sodium fluoride 40 g/l Caustic soda 50 g/l
The operating temperature of the bath is 70-80°C, the processing time is about 1 minute.
Another aqueous solution contains:
Chromium oxide 30 g/l Concentrated sulfuric acid 150 g/l
The operating temperature of the bath is 70°C. Processing time 1-1.5 minutes.
The simplest way to decoratively paint steel products
The electrochemical method can be used to paint steel products in any color. If the paint layer is varnished, it will reliably protect the product from corrosion. The solution in which steel products are painted includes the following components:
Copper sulfate 60 g Refined sugar 90 g Caustic soda 45 g Water up to 1 l
Copper sulfate is dissolved in 200-300 ml of distilled water, then sugar is added to the resulting solution. Separately, caustic soda is dissolved in 250 ml of water and a solution of copper sulfate and sugar is added to it in small portions (with stirring). After mixing these two solutions, add distilled water to 1 liter. The part is cleaned, polished and degreased in a solution used for nickel plating, and then thoroughly washed in warm water. An additional electrode is made from red copper (preferably grades M0, M1). The part and electrode are connected to a flashlight battery (or other 4-6 V DC source), and the copper electrode should be connected to the plus of the battery, and the part to the minus. First, the copper electrode is lowered into the solution, and then the part. After 5-10 seconds, the battery is turned off, and coloring continues without electric power. While in the solution for 2 to 25 minutes, the part is painted in the following colors (in the order of their appearance): brown, purple, blue, cyan, light green, yellow, orange, red-lilac, greenish-blue, green, pink-red . The part can be removed from the solution (checking the color) and put back into the solution - the process will proceed normally. When the part is kept in the solution for more than 25-30 minutes, the process is repeated cyclically many times.
As the electrolyte evaporates, distilled water is added to the bath, since increasing the electrolyte concentration worsens the quality of the color. To obtain more contrasting colors, add 20 g of sodium carbonate (anhydrous soda) to the finished electrolyte. If the painting is unsuccessful, the film can be easily removed by wiping the part with ammonia. The painted parts are washed with water, dried and coated with colorless varnish.
A simple way to decorate an aluminum surface to look like mother-of-pearl
The aluminum surface is cleaned with a metal brush, making small strokes in different directions (creating a certain pattern). Chips and dirt are removed from the surface with a clean rag. A clean aluminum surface is coated with an even layer of 10% caustic soda solution (working temperature of the solution is 90-100°C). After the solution dries, a beautiful film with a pearlescent tint forms on the aluminum surface. For better preservation, the film is coated with colorless varnish. A more beautiful film is obtained if the product or part is heated to 80-90°C before applying the caustic soda solution.
Chemical method of brightening products and parts made of silumin (restoration)
Products and parts made of silumin (an alloy of aluminum and silicon) are quickly covered with an oxide film of dark tones. However, they can remain shiny for a long time if they are lightened. Products or parts are cleaned and, if necessary, polished, then degreased, washed and immersed for 10-20 minutes in the following solution:
Chromic anhydride 100 g Concentrated sulfuric acid 10 g Water up to 1 l
The working temperature of the solution is 18-20°C.
After lightening, the products and parts are washed and dried, and so that the surfaces of the products and parts do not oxidize for a long time, they are coated with colorless varnish.
What you need to know about polishing steel and non-ferrous metals
Polishing is used to improve the cleanliness of the surface of parts and devices, and to eliminate traces of previous processing (strokes, scratches, small dents and tiny irregularities). There are two types of polishing - preliminary and final. Pre-polishing is used to mechanically remove surface irregularities with loose abrasives (in a free state) or grains fixed on the working surface of a polishing wheel. Final polishing is carried out with fine grinding powders or soft elastic wheels with thin polishing pastes applied to them. The finest surface finish is achieved by rubbing a piece of felt or woolen cloth coated with a special metal polishing paste. After polishing, the surface acquires a mirror shine.
Lime paste is used for polishing nickel, brass, aluminum and other metals, its composition (in%) is as follows:
Vienna lime 71.8 Ceresin 1.5 Stearic acid 2.3 Solidol T 1.5 Turpentine 2.2
Composition of paste (in%) for polishing steel and other metals:
Paraffin 20 Stearin 10 Technical lard 3 Micropowder M50 67
Note. Waxy and liquid materials are mixed and heated in a water bath (or over low heat). Then the dry ingredients are mixed into the hot mass.
GOI pastes are intended for polishing steel and other metals and are chromium oxide mixed with waxy substances. Pastes are produced in three grades: coarse, medium and fine. In the absence of chrome paste, you can successfully use chromium oxide oil paint diluted with kerosene. Crocus paste (iron oxide) is sold in stores in ready-made form (in dentures it is used under the name “gold paste”). Crocus paste is used for polishing brass, bronze, silver and other metals. “Shine” powder diluted with machine oil is used for fine polishing of metals.
For recipes for preparing polishing pastes, see the section.
Chemical method of polishing metals
Metals can be polished chemically, i.e. by simply immersing a part or object in a bath of polishing solution without the use of electric current. For this purpose, you can use porcelain glasses or baths. The polishing solution consists of the following substances:
Concentrated phosphoric acid 350 ml Concentrated nitric acid 50 ml Concentrated sulfuric acid 100 ml Copper sulfate or nitrate 0.5 g
The operating temperature of the bath is 100-110°C. Polishing time from 0.5 to 4 minutes. Polishing produces choking fumes, so the bath should be kept in a fume hood or outdoors.
This solution polishes aluminum and its alloys well. It is also suitable for polishing other metals, but the operating conditions (polishing time, temperature) must be different.
CHEMICAL PROCESSING OF METALS
Chemical nickel plating of steel, copper, brass and bronze products
Parts made of steel and copper alloys can be chemically coated with nickel. This coating not only protects parts well from corrosion and gives them a beautiful appearance, but also has increased wear resistance. Another advantage of chemical nickel plating is that nickel is evenly deposited on all, including internal, surfaces of parts.
The part to be decorated with nickel plating must be prepared in an appropriate way: sanded, polished and degreased. Steel parts are degreased in a solution containing 20-30 g of caustic potassium (or caustic soda), 25-50 g of soda ash and 5-10 g of liquid glass (silicate glue) per 1 liter of water; copper - in a solution containing (for the same amount of water) 100 g of trisodium phosphate and 10-20 g of liquid glass. Before nickel plating, copper parts must be kept on the iron for 0.5-1 minutes. It should also be borne in mind that alloys containing more than 1-2% lead or cadmium are not amenable to chemical nickel plating.
Degreasing of steel and copper parts at room temperature ends after 40-60 minutes, at a temperature of 75-85°C - after 20-30 minutes. Then the part is thoroughly washed in running water and immersed for 0.5-1 min in a 5% solution of hydrochloric acid to remove the oxide film, after which it is washed again in water and immediately transferred to the nickel plating solution. 30 g of nickel chloride and 10 g of sodium acetate are dissolved in 1 liter of water heated to 60°C. Then the temperature is brought to 80°C, 15 g of sodium hypophosphate is added - and the solution is ready. The part is immersed in it, the temperature is raised to 90-92°C and maintained at this level until the end of the nickel plating process. At lower temperatures, the speed of the process slows down sharply, and when heated above 95°C, the solution may deteriorate.
The required amount (volume) of solution depends on the area of the part to be nickel-plated. The ratio of this area (in square decimeters) to the volume of the solution (in liters) should be in the range of 2.5-3.5.
So, for example, at S/V=3 in 1 hour the thickness of the nickel layer will be 10 μm.
The chemicals used are not toxic, degreasing and nickel plating are not accompanied by the release of harmful gases.
Chemical copper plating of steel and cast iron parts
Quite easily, copper is chemically deposited on iron, steel and cast iron. The coverage is satisfactory.
To coat these metals, a solution of the following substances is prepared:
Copper sulfate 8-50 g Concentrated sulfuric acid 8-50 g Water up to 1 l
Operating temperature 18-20°C. After thorough cleaning and degreasing, the parts are immersed in the solution for a few seconds. Parts coated with copper are removed from the solution, washed with water and dried.
Chemical chrome plating of metals
Parts made of steel, copper and brass are chemically chrome plated in a solution containing:
Chromium fluoride 14 g Sodium hypophosphate 7 g Sodium citrate 7 g Glacial acetic acid 10 ml Caustic sodium (20% solution) 10 ml Water up to 1 l
Operating temperature is about 80°C. Cleaned and degreased parts are metallized within 3-8 hours. When chemically chrome-plating steel objects, it is recommended to first chemically coat them with copper. Parts with a deposited layer of chromium are washed in water and dried.
Electroless nickel plating of metals
The nickel plating solution consists of the following substances:
Nickel ammonium sulfate 50 g Ammonium chloride 40 g Water up to 1 l
A small amount of zinc metal is added to the solution and stirred continuously.
Chemical dyeing of pewter items bronze
Pewter products can be easily painted bronze using a chemical method. Products are immersed in a solution or wiped with a cloth soaked in a solution consisting of the following substances:
Copper sulfate 25 g Ferrous sulfate 25 g Water up to 500 ml Then the product is dried, cleaned with a brush, wiped with a cloth and again immersed in a solution consisting of the following substances: Copper acetate 100 g Acetic acid 10% 400 ml
After this, the product is dried. If desired, it can be polished and coated with clear varnish.
"Gold plating" of brass
Brass and products made from it quickly tarnish and oxidize in air. To protect highly polished products from oxidation, brass parts are often coated with a special golden varnish. A simpler and more accessible method is as follows: after thorough cleaning and polishing, the brass part is immersed in a 10-15% solution of some alkali to remove fat from its surface. Then the part is washed in water and immersed in a weak (2-3%) solution of sulfuric or hydrochloric acid for 1-2 s. Good results are obtained if brass is dipped in a solution of sodium bisulfite, then rinsed in water and dipped in a solution of copper acetate heated to 36-40°C.
Depending on the time the piece is in the solution, the brass will turn from a light golden color to a red gold color and even a reddish-violet hue. The color of the paint is monitored by removing the part from the solution from time to time. After painting, the part is washed with water and air dried. The color is permanent and does not change over time. Copper acetate is commercially available, but you can make it yourself. To do this, you need to dissolve 5 g of copper sulfate in 0.5 liters of water, then mix it with a solution of lead acetate (pharmacy lead lotion or lead sugar).
The second solution is made up of 8 g of lead acetate and 0.5 liters of water. When mixing the solutions, a precipitate of lead sulfate precipitates, and copper acetate remains in the solution. This solution will serve as the working solution. The precipitate can be filtered or left at the bottom of the vessel.
Copper gold coloring
4 g of caustic soda and 4 g of milk sugar are dissolved in 100 g of water, boiled for 15 minutes, then with constant stirring, 4 g of a solution of saturated copper sulfate is added in small doses. Well-cleaned copper products are immersed in the hot mixture. Depending on the duration of action, they acquire different colors - from gold, green to complete black.
Golden varnish for brass (passivation of brass)
When brass is passivated, a stable protective film similar to gold plating is formed. This film is not afraid of moisture, so fishermen passivate brass lures. The cleaned, polished and degreased part is dipped for 1 second in a solution prepared from 1 part nitric and 1 part sulfuric acid, and immediately transferred to a strong solution of potassium dichromate (chrompic) for 10-15 minutes.
After this, the part is washed and dried.
Chemical staining of brass
The cleaned, degreased and washed part is dipped into one of the following solutions.
1st solution: Hyposulfite 11 g Lead sugar 39 g Water up to 1 l Solution temperature 70°C.
2nd solution: 10 g of sodium hydroxide and 10 g of milk sugar are dissolved in 250 ml of boiling water. Then, stirring continuously, add 10 ml of a concentrated solution of copper sulfate to the solution.
Within 3-10 minutes, the part in one of the solutions turns golden, bluish, blue, violet and, finally, rainbow.
When the desired color is obtained, the part is removed, dried and polished with cloth.
Brass acquires a bluish-black color when the prepared part is immersed for 1-3 minutes in the following solution:
Ammonia (25% ammonia) 500 ml Copper bicarbonate (or carbon dioxide) 60 g Brass (sawdust) 0.5 g
After mixing the components, the solution is shaken vigorously 2-3 times, after which the part is immersed in it.
Brass turns brown when the part is immersed in one of the following solutions.
1st solution: Hyposulfite 50 g Copper sulfate 50 g Water up to 1 l Solution temperature 70°C.
2nd solution: Sodium sulphide 100 g Water up to 1 l Solution temperature 70°C.
3rd solution: Lead acetate 30 g Hyposulfite 90 g Water up to 1 l Solution temperature 80-90°C.
To prepare the 3rd solution, you need to dissolve both substances separately in half the volume of water, then drain them together and heat to 80-90°C. After painting, the part is washed with warm water, dried and coated with colorless varnish.
A simple method of silvering
Spent hyposulfite (fixer) is used as a silvering compound, which is no longer suitable for fixing photographic films or photographic paper. The method is extremely simple. The copper part is cleaned to a shine, boiled in a soda solution and thoroughly washed with water. Then it is dipped into used hyposulfite. After some time, silver will settle on the part. After washing with water, the part is dried and polished with cloth. The quality of silver plating and the strength of adhesion of silver to copper depends on the concentration of silver in the hyposulfite solution.
Hot silvering of metal parts
Any metal can be silvered using this method. It consists of the following: a cleanly processed part is immersed on a zinc strip in a boiling solution consisting of the following components:
Potassium iron sulfide 120 g Potash 80 g Silver chloride 7.5 g Distilled water up to 1 l
The silvering process ends after the surface of the part is completely covered with silver. The part is then removed from the solution, washed and polished. It should be remembered that when the solution boils, harmful substances are released, so boiling should be done in the open air or under a hood.
Chemical silver plating
1. Several sheets of Unibrom matte photographic paper are cut into pieces and dipped into a solution of fixing salt (the salt is diluted in the volume of water indicated on the package).
The cleaned and degreased part is placed in this solution and rubbed with an emulsion layer of paper until a dense layer of silver is formed on the surface of the part. After rinsing in warm water, wipe the part with a dry cloth.
2. To 300 ml of used fixer (remaining after printing photographs), add 1-2 ml of ammonia and 2-3 drops of formaldehyde (the solution is stored and worked with only in the dark).
The cleaned and degreased part is placed in the solution for 0.5-1.5 hours, then washed in warm water, dried and wiped with a soft cloth.
Paste for silvering
Parts made of copper, bronze, brass, and copper-plated iron can be plated with silver using pastes.
1. The paste for silvering is prepared as follows: in 300 ml of distilled water or water obtained from ice in household refrigerators, dissolve 2 g of silver nitrate (lapis) and add a 10% solution of table salt to the solution until the precipitation of chloride sediment stops. silver This precipitate is washed 5-6 times in running water. Separately, 20 g of hyposulfite and 2 g of ammonium chloride (ammonia) are dissolved in 100 ml of distilled water. Then silver chloride is added to the resulting solution in small doses until it stops dissolving. The resulting solution is filtered and mixed with finely ground chalk to the consistency of thick sour cream. The pre-degreased part is rubbed with a paste using cotton wool or gauze until a dense layer of silver is formed on its surface, after which the part is washed with water and wiped with a dry rag.
2. The polished and degreased part is rubbed with a cloth or a piece of soft leather, onto which a paste of the following composition is applied:
Silver chloride 6 g Table salt 8 g Potassium tartrate (tartar) 8 g
The listed substances are ground in a mortar and stored in a dark container; before use, the mixture is diluted with distilled water to obtain a liquid paste. When the part is covered with a layer of silver, it is washed in water and rubbed until shiny with soft flannel.
3. The paste for silvering is prepared as follows: pour 2 g of ammonia, 4 g of tartar and 1 g of silver nitrate (lapis) into a vessel, add a little distilled water until a semi-liquid slurry is obtained. Then, with a cloth with paste applied to it, the polished and degreased part is rubbed to a silver shine.
Chemical method of silvering non-metallic materials
Non-metallic parts, such as plastics, glass, ceramics, wood, etc., can also be metalized using a chemical method. The solution given below for silvering non-metallic materials gives very good results, especially when metallizing glass (silvering of mirror surfaces, vessels, incandescent lamp bulbs, reflectors for projection equipment, etc.).
The composition of the silvering bath includes the following substances:
Composition A Silver nitrate 12 g Ammonium nitrate 18 g Distilled water 500 ml After complete dissolution of the substances, the solution is added with distilled water to 750 ml.
Composition B Caustic soda (chemically pure) 19 g Distilled water 500 ml After complete dissolution of caustic soda, the solution is added with distilled water to 750 ml.
Composition B Sucrose 12.5 g Tartaric acid 1.5 g Distilled water 125 ml The solution is boiled for 20 minutes and then topped up with distilled water to 500 ml.
All solutions are stored separately in dark containers with ground-in stoppers.
The solution for silvering is obtained by mixing compositions A and B B is added immediately before silvering . Parts intended for silvering are thoroughly cleaned in a hot soda solution, rinsed with running water and immersed in a bath with freshly prepared solution. The working temperature of the solution is 18-20°C. Silvering time - 10 minutes. Metallization can be carried out two or three times in succession, but each time in a fresh solution. Silver-plated parts are dried at a temperature of 50°C for 1 hour, and at a temperature of 18-20°C for 24 hours. The silver layer can be easily removed from glass, porcelain or ceramics with nitric acid.
Chemical dyeing of silver objects purple
Silver or silver-plated objects acquire a purple color in a solution consisting of the following substances:
Anhydrous sodium sulfate 12.5 g Sodium carbonate 5 g Water 500 ml
The solution is heated to 80°C and the object is immersed in it for a few seconds. The item is then allowed to dry. The surface of the object can be coated with transparent varnish.
Chemical solution for dyeing silver objects black
Silver or silver-plated objects become black after boiling them in a solution of sodium sulfate (100 g per 500 ml of water). After boiling in this solution, the objects are dried and coated with a clear varnish.
Hot gilding of metal products
Mix 20 g of nitric and 20 g of hydrochloric acid in a glass vessel. 1 g of gold is dissolved in this mixture. When the gold dissolves, 1 g of antimony chloride and 1 g of pure tin are added to the solution. The vessel with the solution is placed in hot water and boiled until the tin dissolves, after which 20 g of a saturated boric acid solution is added. Products intended for gilding are cleaned, polished and boiled in a solution of caustic potassium or soda. The solution is applied to the product with a brush; The dried product is heated over the flame of an alcohol lamp or over a charcoal fire. After heating, a good gilding is obtained that does not require polishing. Store the solution in a glass container with a ground-in stopper in a dark place.
Gold plating without external power source
Contact gold plating is used to obtain very dense and uniform coatings, characterized by high adhesion strength, and if a large coating thickness is not required. Electrolysis by this method does not require an external current source. The potential difference required for gold deposition is created by a galvanic cell, in which the cathode is the coated product, immersed in a gold-plating electrolyte, and the anode is a zinc plate located in a concentrated solution of table salt and connected to the product with a wire, as shown in Fig. 1.
For electrolysis, any heated gold-plating electrolyte from those indicated in the table can be used.
Compositions of solutions and operating modes for gilding using the immersion method
Gilding by immersion is based on the creation of a potential difference at the boundary of the surface of the metal being coated and the adjacent electrolyte layer. Good quality coatings are formed only on brass or brass plated parts. Therefore, parts made of other metals are pre-brass-plated (minimum layer thickness 1-2 microns). The gilding process automatically stops when a gold layer about 0.1 microns thick is obtained, but the coating is dense, glossy and has good adhesion to the surface of the parts.
Removal of poor-quality gold coatings
To remove poor-quality coatings, gold-plated silver items are suspended as anodes in a 5% solution of hydrochloric acid at a temperature of 18-20°C. Iron or lead plates serve as cathodes. Anodic current density 0.1 - 1 A/dm2. Copper pendants. In addition, the gold coating can be removed in aqua regia. "Royal vodka" is a mixture of acids (50% nitric acid mixed in 50% hydrochloric acid). The mixture is used for etching copper, brass, iron, steel, zinc, etc. This solution acts on metals almost instantly; Corrosion and dirt disappear and the metal surface becomes shiny or, more often, matte. Jewelers use this mixture to determine pure gold.
Note. When using active acids, safety rules must be strictly observed. It should be remembered that when diluting an acid with water (for example, sulfuric acid), you need to pour the acid into the water, and not vice versa, since otherwise the acid will splash, which can lead to severe burns.
Simple methods for extracting silver from waste hyposulfite (fixer)
Only part of the silver contained in the photosensitive layer of the photographic material is consumed to construct a photographic image. Most of the silver goes into the fixer and developer; it can be isolated and collected.
1st method. Allows you to highlight pure silver. It consists of the following: iron filings or small iron nails, well washed from grease with gasoline, are poured into a vessel with depleted fixer. Shake the solution from time to time. After 7-10 days, the solution is drained and the nails are dried in air. Silver deposited on nails falls off as a black powder, which can then be smelted into ingots.
2nd method. The depleted fixer and an equal volume of spent metholhydroquinone developer are poured into one vessel. A 30% solution of sodium hydroxide is added to the resulting mixture at the rate of 100 ml for each liter of used fixer. The silver is deposited in the form of the finest pure silver powder. The process lasts at least 48 hours. The silver precipitate formed during this time is filtered out and dried. The remaining aqueous solution of sodium thiosulfate, i.e. fixer, can be used again in work.
3rd method. A polished sheet of brass is placed in the used fixer, which is in a glass vessel. After 48 hours, almost all the metallic silver from the depleted solution will have deposited on it. After deposition, the sheet is washed well with water and dried. Then the layer of silver is carefully scraped off its surface.
4th method. To 1 liter of used fixing solution add 5-6 g of sodium hydrosulfite and 5-6 g of anhydrous soda. After 19-20 hours, the metallic silver formed in the form of a black fine powder is filtered, and the desilvered fixing solution is acidified with sodium bisulfite and used again for work.
5th method. To do this, prepare a 20% solution of sodium sulfate and pour it into the used fixer at the rate of 20 ml of solution for each liter of fixer. After thoroughly mixing the solution, it is allowed to stand for 24 hours. Then the solution is drained from the sediment, and the sediment is dried on paper. The precipitate is silver sulfide. Precipitation is carried out in the open air or with increased ventilation; to reduce the release of hydrogen sulfide, the spent fixing solution is pre-alkalinized.
Note. For more information on methods for obtaining silver from various wastes, see the collection: “Home technologies for obtaining silver, gold, platinum... - from waste.” The resulting silver can be used in the practical tips (recipes) found in this article.
COLORING OF METALS
Coating metal with moiré varnish
Before coating with “moire” varnish, the surface of the metal part is degreased by heating it in an oven (oven) for 15-20 minutes at a temperature of 80-100°C, then primed with heat-resistant enamel, puttied with varnish putty and dried. When the part is thoroughly dry, it is treated with pumice with water and sandpaper, wiped dry, coated with an even layer of “moire” varnish using a spray bottle and placed for 10-15 minutes in an oven at a temperature of about 80°C.
The pattern of the pattern depends on the thickness of the coating and the duration of heating of the part. Once the pattern has formed on the part, it is removed from the oven for a short time to allow partial cooling, and then placed back into the oven to final dry the varnish. At a temperature of 120-150°C, the varnish completely dries within 30-40 minutes, and at a lower temperature - within 2-3 hours.
To protect the painted surface from dust, it is coated with celluloid varnish: celluloid is dissolved in acetone to the consistency of liquid oil varnish and applied to the surface in an even layer using a swab. After the acetone dries, a strong protective film remains on the surface.
Painting steel products to look like aluminum
To give steel products a beautiful look and protect them from corrosion, the metal is often coated with aluminum paint - a varnish containing aluminum powder. To do this, 15 g of powder is poured into colorless nitro varnish diluted with acetone (110 g).
In the same proportion, the paint can be diluted not in nitro varnish, but in celluloid glue - acetone, in which 5-10 g of X-ray film, cleared of the emulsion, is dissolved.
The surface of the product is first thoroughly cleaned and then a thin layer of paint is applied using a spray gun.
A durable coating is obtained if BF-2 glue is added to aluminum paint. BF-2 glue is dissolved in alcohol until the enamel becomes thick, then dry aluminum powder is poured into the resulting solution and mixed thoroughly, after which alcohol is added again until normal viscosity is obtained.
The paint prepared in this way adheres well when painted with a brush or with a spray gun, it does not crumble and retains its appearance for a long time.
What you need to know about paint incompatibility and the peculiarities of paint color perception
All paint components are chemicals. Metals (copper, zinc, aluminum), which are part of paints in the form of powder, affect the corrosion of the metal surface being painted and the binder. Metal oxides and salts affect the binder, accelerating film formation. Dissimilar types of binders cannot be combined with each other, and some oil paints made with the same binder, but based on different pigments, cannot be mixed.
Pigment incompatibility. When mixing pigments, it is very important to consider the nature of their interaction. If pigments are incompatible, they are destroyed and their anti-corrosion properties are lost.
When mixing paints with incompatible pigments, their color is lost.
Incompatibility of binders. You can mix oil paints only with oil paints (on a homogeneous basis), glyphthalic paints with glyphthalic paints, pentaphthalic paints with pentaphthalic paints, epoxy paints with epoxy paints, bitumen varnishes with asphalt and coal tar varnishes, etc. However, all thick oil paints can be diluted with drying oils and varnishes made on the basis of only light natural and artificial resins, excluding asphalt and bitumen resins.
Incompatibility of paint with surface material. All primers without exception can be applied to a steel surface: oil, phosphating, tread, glyphthalic, phenol-formaldehyde, vinyl chloride copolymers, ethylene, acrylic, etc.
The following primers cannot be applied to an aluminum surface: lead (lead white, lead, red lead), red lead, verdigris and cinnabar, no matter what binder they are used on. You can apply phosphating primers VL-02 and VL-08; glypthal KF-030, GF-031, GF-032; acrylic AG-10S and epoxy E-4021 and EP-09T yellow.
Peculiarities of paint color perception.
Colors are perceived as “warm” or “cool”. Visually, they can bring a painted object closer or further away. The perception of different colors is shown in the table.
Author-compiler. Patlakh V.V. 2004
» » .. 1993-2007 .
Electrolyte preparation
After making sure that all the necessary varnish has been removed, we proceed to preparing the working solution. During this time the protective layer will have time to dry completely. As a substance in which steel products are etched, a solution of table salt is most often used at home. To prepare it, you need to dissolve the crystals in clean water in a ratio of two tablespoons of salt per 0.5 liter of liquid.
Instead of sodium chloride, you can use another quite accessible chemical called copper sulfate. It is not difficult to purchase at any hardware or garden store.
Blackening of brass, one of the methods
Recipe taken from here
Additional material on the topic: One of the methods for blackening brass
Let me make a reservation right away that I am not a chemist, and all my knowledge is only what I have left over from school. Therefore, the recipe was not fully followed and some deviations were made.
Blueish-black color
| 25% ammonia solution (ammonia) | from 100 g/l to 1 l |
| Copper carbonate (CuCO3) | 40-200 g/l |
Treatment lasts 25-30 minutes. at a temperature of 15-30°C.
Preparation of copper carbonate.
In this case, when recipes mention copper carbonate, in fact, they are usually talking about basic copper carbonate. To obtain it, you can use copper sulfate, acetate or nitrate.
A solution of potassium or sodium carbonate (potash, soda) is added to an aqueous solution of one of these salts.
The green-blue precipitate that falls consists of basic copper carbonate of variable composition, nCuCO3 x mCu(OH)2, where n and m can vary within some limits, which for us, in this case, is not very important. The precipitate is filtered off, washed with water and can be used.
The process itself.
To blacken brass using this method you will need:
- copper sulfate;
- baking soda;
- ammonia;
- two glass jars, preferably the same volume;
- filter paper or something similar;
- stirring stick.
The vitriol was purchased at a nearby hardware store in the department that sells all sorts of pesticides for controlling garden pests. Soda - in the nearest grocery store, where there is simply plenty of it.
Ammonia - in the nearest pharmacy. But only 10%. As they explained to me, 25% is not for sale due to its danger.
| First we prepare copper carbonate. For this we need copper sulfate and ordinary soda. |
| Dissolve the vitriol in warm water. The solution needs to be saturated, i.e. add vitriol until it still dissolves. The solution must be made so that it occupies only half the container. |
| Then slowly, a little at a time, add soda to the saturated vitriol. It is precisely because the solution begins to bubble like soda that I recommend making vitriol in only half the container. |
| Stir. Over time, the seething subsides. I poured in soda until the bubbling stopped. After a while you will see that the result is a fine suspension of a pleasant turquoise color. On the surface you can see that the fry are still seething there. |
| Now the resulting suspension must be filtered. There was nothing worthwhile at hand except napkins. The recipe says to dry it. But I didn't do this. |
| Now we need a 25% ammonia solution. As I already wrote, only 10% is available in the pharmacy. This is what we will use. |
| Little by little add turquoise porridge to ammonia. The solution turns blue. |
| We also need a saturated solution. Therefore, we add, without sparing, copper carbonate. You will end up with a blue-black liquid. This is what we need. |
The solution is ready, all that remains is to put the necessary parts in there. Blackening time, according to the recipe, is 25-30 minutes.
After removing the part, rinse with water and dry/wipe.
This is the final result (blue-black, blued steel color):
Some notes:
- Vitriol is not suitable for impregnating wood against fungi;
- It is convenient to use two containers, because you will have to filter the solution;
- You only need to prepare half a container of vitriol, because then there will be a violent seething;
- You can also use 10% ammonia;
- If you rinse the copper carbonate well, the reaction proceeds much faster;
- Flat parts blacken faster than rings, nails, eyelets and the like;
- If the part is kept in the solution, copper carbonate will settle on it in the form of turquoise crystals, which, in principle, can then be easily scraped off with a fingernail without damaging the blackening;
- If you have difficulty removing blue sediment from a part, I recommend simply heating it in the flame of a candle or lighter. The sediment will immediately fall off on its own.
from www.shipmodeling.ru
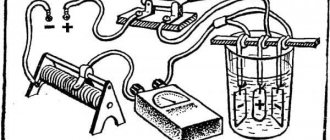
Etching a metal product
To activate the process, you must place any steel object in the electrolyte solution and connect a negative wire from a current source to it. The positive wire in this case is connected to the workpiece. Schematically the etching process looks like this:
In the absence of a high-quality converter (rectifier), you can use a phone charger by cutting off the contact for the corresponding socket.
The etching process under the influence of electric current occurs quite rapidly.
This must be taken into account when choosing a glass container. The electrolyte level in it should prevent its possible splashing out during the etching process.
Control of pattern deepening during electrochemical processing can be carried out visually by periodically removing the workpiece from the solution. If all necessary parameters are met, the process can last up to several minutes, depending on the desired depth of etching of the pattern on the metal part.
Once you get the desired result, turn off the electric current. After this, carefully remove the varnish film from the extracted sample. To do this, it is convenient to use nail polish remover and a thick cloth. Wash the finished drawing with warm water and soap.
Basic metal etchant solutions
1. Carbon steels are treated with an 8–20% solution of sulfuric acid or a 10–20% solution of hydrochloric acid. To avoid further fragility of the material and reduce the likelihood of over-etching, corrosion inhibitors are added to the composition, such as KS, ChM, UNIKOL.
2. Stainless or heat-resistant steel is pickled with a mixture of 12% hydrochloric, 12% sulfuric, 1% nitric acid. If necessary, processing is carried out in stages. Then, using 20% hydrochloric acid, the scale is loosened, after which the product is dipped in a 20–40% nitric acid solution to remove surface contaminants.
3. From stainless steel, a thick layer of scale formed during the production process is removed with a 75–85% solution of caustic soda in combination with 20–25% sodium nitrate. Next, the oxides are removed by etching the metal with 15–20% nitric acid.
4. Aluminum and its alloys are cleaned from the oxide film located on the surface of the workpiece using alkaline or acidic solutions. Most often, 10–20% alkali is used, all exposure occurs at a temperature of +50…+80 °C for at least two minutes. Sometimes sodium chloride and sodium fluoride are added to the alkali to achieve more uniform etching.
5. Titanium and alloys based on it are purified in several stages after heat treatment. First of all, it is necessary to loosen the scale in concentrated caustic soda, then it is removed in a solution of sulfuric, nitric or hydrofluoric acid. At the final stage, the remaining pickling sludge is disposed of using hydrochloric or nitric acid with a small addition of hydrofluoric acid.
6. Copper and its alloys are treated with hydrogen peroxide, chromic acid and a number of salts, such as:
- copper chloride;
- ferric chloride;
- ammonium persulfate.
Getting a color drawing
To give the resulting pattern, drawing or inscription an additional decorative effect, we suggest using a simple method accessible to everyone. To do this, apply a small amount of any nitro enamel to the treated area, achieving complete filling of the recess. This paint and varnish material dries quickly enough, so you can return to further work after an hour. After making sure the enamel is completely dry, remove any excess material that has fallen onto the surface of the etched piece using fine sandpaper. The use of a solvent in this case can ruin the entire work, since it smears the paint over the surface without completely removing it, and can give the pattern an unattractive dullness.
Mechanical polishing of the finished product on a felt wheel with GOI paste will give the painted etched pattern the final expressiveness.
Dear readers, comment on the article, ask questions, subscribe to new publications - we are interested in your opinion
Why should you contact us?
We treat all clients with respect and carry out tasks of any size equally scrupulously.
Our production facilities allow us to process various materials:
- non-ferrous metals;
- cast iron;
- stainless steel.
When completing an order, our specialists use all known methods of metal machining. Modern equipment of the latest generation makes it possible to achieve maximum compliance with the original drawings.
In order to bring the workpiece closer to the sketch submitted by the customer, our specialists use universal equipment designed for jewelry sharpening of tools for particularly complex operations. In our production workshops, metal becomes a plastic material from which any workpiece can be made.
The advantage of contacting our specialists is their compliance with GOST and all technological standards. Strict quality control is carried out at every stage of work, so we guarantee our customers a conscientiously completed product.
Thanks to the experience of our craftsmen, the output is an exemplary product that meets the most demanding requirements. At the same time, we start from a strong material base and focus on innovative technological developments.
We work with customers from all regions of Russia. If you want to place an order for metalworking, our managers are ready to listen to all the conditions. If necessary, the client is provided with free specialized consultation.
LiveInternetLiveInternet
-Music
—Tags
—Categories
- handicrafts (5341)
- knitting (1796)
- embroidery (1211)
- magazines (813)
- costume jewelry (603)
- beads (514)
- handmade (440)
- toys (416)
- Fashion (281)
- plastic (272)
- quilt (97)
- tapestry (66)
- macrame (45)
- decoupage (35)
- decoupage (15)
- quilling (10)
- cooking (1888)
- Baking (620)
- Vegetarianism (524)
- preparations for the winter (335)
- cake (144)
- art (1881)
- music (507)
- painting (351)
- drawing (220)
- antiques (201)
- painting (184)
- jewelry art (183)
- sculpture (163)
- artist (163)
- museums, exhibitions (39)
- batik (21)
- Master classes (1441)
- Videos (815)
- countries and peoples (742)
- Indians (123)
- China (74)
- India (50)
- Buryats (28)
- Evenki (4)
- self-development (669)
- Psychology (475)
- photos (462)
- house (406)
- interior (249)
- Feng Shui (5)
- gardening (362)
- health (325)
- BEAUTY OF THE NORTHERN REGION (311)
- Interesting (302)
- Great Rus' (292)
- Baikal (258)
- humor (234)
- esoterics (190)
- home cosmetics (168)
- Our smaller brothers (142)
- thinking out loud (121)
- Severobaykalsk (95)
- holidays (90)
- cats (85)
- Yoga (80)
- PORCELAIN (77)
- religions (77)
- Buddhism (42)
- my home (76)
- my creativity (45)
- My family (18)
- store (4)
- hippie (67)
- gems (44)
- people (44)
- Secrets of the Earth (44)
- vintage (33)
- mandalas, fractals (31)
- history (22)
- Universe (3)
-Video
—Photo album
—Search by diary
—Subscription by e-mail
-Friends
— Regular readers
-Statistics
Glossy paper
In addition to glossy paper (you can buy it at art supply stores, or you can simply cut a sheet out of a magazine), you will need a laser printer, an imaging application, and an iron. The image of the drawing should be mirrored and printed in full size. The image is applied to the surface and ironed several times. After the workpiece has cooled, the paper is washed off with warm water, and the toner remains on the surface of the part. The back and side surfaces that are not subject to etching must be protected with varnish or plasticine.
The main advantage of the method is that the smallest details of the image can be accurately transferred.
The main disadvantage is that you can only work in this way with flat or cylindrical workpieces. The method is very popular in the manufacture of printed circuit boards.
Electrochemical etching of metals.
Electrochemical etching of metals.
#1 Post by IGOR » Fri Feb 05, 2010 8:07
Re: Electrochemical etching of metals.
#2 Post by FreeLander » Fri Feb 05, 2010 9:17
Re: Electrochemical etching of metals.
#3 Post by IGOR » Fri Feb 05, 2010 13:31
Re: Electrochemical etching of metals.
#4 Post by FreeLander » Fri Feb 05, 2010 15:07
Re: Electrochemical etching of metals.
#5 Post by IGOR » Fri Feb 05, 2010 15:22
Re: Electrochemical etching of metals.
#6 Post by frog » Fri Feb 05, 2010 17:17
Re: Electrochemical etching of metals.
#7 Post by Hedgehog » Fri Feb 05, 2010 20:19
You can do anything!
In short, using the electrochemical method: - electrolyte - a saturated (the powder no longer dissolves) solution of copper sulfate; - the current goes “from the excess (plus (anode)) to the deficiency (minus (cathode). Therefore, the board is the anode, the electrode is the cathode. - the cathode material is any (I have 3 mm lead), since it is not consumed. - distance from the board to the electrode - 30-50 mm. By the way, the distance and current can be successfully adjusted. - current density (for me) is in the range of 1-2 A per sq. dM. It must be selected in each specific case. - it gets hot, but not much , a plastic jar will hold up (my 8 mm plexiglass bathtub has been in service for three years) It is very important (!) that the electrode(s) and the board should be as flat as possible and as parallel to each other as possible.
By the way, after re-reading the old thread. Over the past 2+ years, my outlook on life has become somewhat simpler. Now, for simple one-time work on thin copper and brass, I again actively use ferric chloride. Yes, the quality is poor, but the speed of obtaining the result makes up for everything. There are two key points - the temperature of the solution (40-60 C), active mixing (aquarium spray) and the process is completed in 10-15 minutes. There is no need to specially heat the solution; it is enough to dilute ferric chloride in hot water, the residual heat will be enough for at least half an hour.
Good luck to everyone. Dmitriy.
Experts are like dirt. There are not enough modelers. Don't tell me what I'm doing wrong. Show me how you do it. Full-time school of classical ship modeling: Here, on the forum, Facebook
Re: Electrochemical etching of metals.
#8 Post by IGOR » Fri Feb 05, 2010 20:52