Article updated and supplemented: May 30, 2021
The surface of the stainless steel has a protective layer that can be damaged by welding. To restore it and in order to protect the surface of stainless steel, including welds, from destruction due to corrosion and rust, a special treatment is used - passivation. As a result, a layer is formed that is resistant to external influences. If the quality of passivation of stainless steel welds is high, then an even protective layer
, showing equal density in different areas.
Metal passivation technology, types and compositions
Passivation is the formation of thin oxide or salt films on the metal surface that protect it from external corrosion.
This coating prevents metal from coming into contact with oxygen and aggressive environments. During passivation, protective films can form on a metal surface both naturally and artificially. In the first case, they consist of oxides of chemical elements that are part of the metal itself, and in the second, they may include oxides and salts of other chemical elements. For example, pure aluminum naturally forms a very durable oxide film and is therefore resistant to most types of corrosion. But products made from its alloys, containing chemically active components, already require artificial corrosion protection and therefore undergo passivation in saline solutions. Passivation is widely used to protect the surfaces of products made of steel, copper, nickel, aluminum and their alloys. Even protective zinc and cadmium coatings are passivated with chromium salts to increase their corrosion and mechanical resistance. Passivation of a metal causes the formation of a layer of oxides or salts several microns thick on its surface, which practically does not affect the geometric dimensions of the products. On the other hand, such films can reduce the contact conductivity of the base material, but, as a rule, to a lesser extent than a layer of corroded metal.
Types of corrosion
Despite the fact that the corrosion process leads to almost the same consequences, the reasons that cause it may be different. The most common cause of corrosion of stainless steel products used in domestic conditions is the use of cleaning products containing a significant amount of chlorine in their chemical composition. Such agents actively contribute to the destruction of the oxide film on the metal, which leads to the development of a corrosion process over its entire surface (i.e. general corrosion).
Crevice corrosion of stainless steel occurs in cases where parts made of such metal come into contact with each other for a long time. Corrosion of this type, which is typical, often begins to develop in the fastening areas. There is also pitting corrosion, which is often called pitting. It occurs when the oxide film on stainless steel is damaged mechanically.
Corrosion of stainless steel under water manifests itself to a greater extent at the joints of parts
If stainless steel comes into contact with a dissimilar metal in a conductive environment, corrosion begins to develop, which is called galvanic. Products made of stainless steels that are used in sea water and at the same time in contact with metals with a lower degree of alloying are most susceptible to this process.
Intergranular corrosion is a very common phenomenon that occurs when a stainless steel product has been subjected to significant overheating. With strong heating (over 500°), chromium and iron carbides are formed at the boundaries of the crystal lattice of stainless steel, which cause a decrease in the strength of the metal.
Corrosion of stainless steel can occur due to the use of chlorine-containing cleaning compounds
There is also erosive corrosion, which occurs if stainless steel is constantly exposed to an abrasive environment. Constantly affecting the metal surface, particles of such a medium destroy the protective oxide film, which does not have time to recover.
The essence and description of the metal passivation process
When passivating, the surfaces of metal products are treated with solutions of chemical compounds that have oxidizing properties. This role is most often played by acids, nitrites and solutions of chromium salts (less commonly, molybdenum). The solution is applied to the surface of metal workpieces by immersion or manually using special equipment. Solutions used for passivation usually consist of a main reagent and several additives that accelerate and stabilize the passivation process.
In general, the passivation process consists of the following stages:
- Mechanical cleaning of product surfaces.
- Chemical degreasing in a solution of sodium hydroxide and soda ash.
- Rinse in running hot and then cold water.
- Passivation for a specified time.
- Neutralization in soda ash solution.
- Rinsing by repeated immersion in running cold water.
- Dry in a drying cabinet or blowing warm air.
- Surface quality control after passivation is carried out visually or instrumentally. If the result is unsatisfactory, the passivation process is repeated, starting from step 1.
The given example describes the technological process of passivation using stationary production equipment. To passivate the surfaces of products at the site of their installation, hand-powered tools and devices are used (see photo below).
Properties of passivated metal and its application
After passivation, a corrosion-resistant layer is formed on the metal surface, which, if chromates are used, also has increased mechanical strength. Some metals and alloys are prone to natural passivation. This is especially true for aluminum and stainless steel with the presence of chromium. But if the structure and chemical composition of the surface layer is damaged, they can also be subject to corrosion. When passivating stainless steel, its own chromium is used to create a durable surface protection, which, when combined with oxygen, forms a dense oxide film. All stainless steel products operating in aggressive environments are pre-passivated, which helps to avoid (or delay) their corrosion.
Passivation of iron and its alloys in the form of structural and special steels is usually carried out over a coating of nickel, zinc or cadmium using chromium salts. This passivation strengthens the surface layer and allows steel products to be used for a long period without the danger of corrosion, and if it occurs, only the affected areas should be treated. Passivation of copper and its alloys (bronze and brass) is carried out for both protective and decorative purposes using chromate solutions. In this case, a thin transparent film is formed on the surface of the copper product, protecting the metal from oxidation and preserving its presentation.
Passivation of silver is carried out for the same purposes using similar technologies.
What causes the high corrosion resistance of stainless steels?
The essence of such a phenomenon as corrosion is that the surface of the metal begins to deteriorate under the influence of negative external factors and the environment. Typically, corrosion due to constant oxidation affects the metal layer by layer, gradually destroying the internal structure of the steel. In many cases, it no longer makes sense to localize the affected areas of the internal structure of the metal, so steel products have to be replaced with new ones.
Passivation (or passivation), as a technology that allows for reliable protection of steel from corrosion, underlies the creation of such a unique metal as stainless steel. The chemical composition of the vast majority of steels belonging to the stainless category may contain various elements:
However, the main alloying element of such steels, the amount of which in their composition can vary between 12–20%, is chromium. The addition of various alloying elements to the composition of stainless steels makes it possible to give them the required physical and chemical characteristics, but it is chromium that is responsible for the corrosion resistance of the steel alloy.
The effect of chromium on the properties of stainless steel
Stainless steel alloys, which contain 12% chromium, exhibit high corrosion resistance only when interacting with ambient air. If the amount of chromium in the chemical composition of stainless steel is increased to 17%, then products made from it can easily interact with nitric acid without losing their performance characteristics.
To make the metal resistant to even more aggressive environments, which include hydrochloric, sulfuric and other acids, the quantitative content of chromium in it is not only increased, but also elements such as copper, molybdenum, nickel, etc. are added to its composition. In other words , they passivate the metal, that is, increase its passivity to corrosion processes.
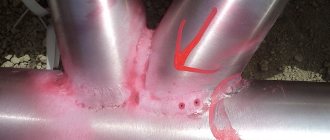
During the process of passivation of the weld zone, a strong film is formed
Passivation, in which appropriate alloying elements are added to the chemical composition of stainless steel, is not the only condition for high corrosion resistance of the metal. In order for the protective properties of stainless steel to remain at a high level, the oxide film on its surface, consisting mainly of chromium oxide, must be intact, have a uniform chemical composition and thickness.
Types of passivation
According to the coating application method, passivation is of two types: chemical and electrochemical. In addition, varieties of this technology are classified according to the type of chemical element from the compounds of which the surface film is formed (chromatization, nickel plating, molybdenation, and others). In addition, natural passivation is distinguished - the process of formation of a protective layer in a number of metals and alloys under the influence of atmospheric and dissolved oxygen in water.
Chemical
Chemical passivation occurs as a result of the attraction of negative ions of salts dissolved in water to the surface of the metal, the atoms of which have a positive potential. To do this, metal products, previously cleaned and degreased, are placed in a special bath filled with an appropriate solution. The main component in such an electrolyte is a metal salt that forms a protective film on the surface of the product. Chemical passivation can also be performed at the site where the product is installed. In this case, all processes, from cleaning to passivation, neutralization and washing, are performed manually using special equipment.
Electrochemical passivation of stainless steel seams
One of the ways to passivate stainless steel welds is the electrochemical method
. According to the technology, the heat-affected zone is exposed to electric current and specially developed electrolytes for cleaning, etching, passivation and polishing of stainless steel. When current flows through a solution, changes occur in the chemical composition of the substances involved in the reaction.
When removing tarnish from stainless steel seams, the surface does not deteriorate, this means that if you use “mirror” steel, then after processing you will not see dull, dull spots in the heat-affected area, which can remain when using nitrogen-containing etching pastes. Also, during electrochemical passivation of stainless steel welds, nothing happens to either the matte or polished surface. Moreover, after processing the seams using this technology, the alloying (passive) layer of stainless steel is completely restored, which during further operation protects the steel surface from corrosion.
Apparatus for passivation of welds
Stainless steel welds can be cleaned and passivated using special equipment, one example of which is the SteelGuard series welders.
. These units are designed to perform cleaning of stainless steel welds, including the final functions of passivation and polishing of stainless steel seams.
Equipment such as the SteelGuard 685 electrochemical seam cleaner allows you to control the required current strength, so you can work effectively on any type of surface without fear of damaging the metal when touching a bare electrode, which previously left an irreparable mark and spoiled the surface of stainless steel.
Our company’s case study of replacing the chemical method of passivation of welds with an electrochemical method in food production can be seen in the article “Apparatus for passivation of welds in food production PTK NIKA.”
Contents of passivation compositions
The composition of solutions for passivation of non-ferrous metals most often includes potassium and sodium chromates, as well as chromic anhydride as the main reagent. To create an acidic environment, various acids and salts are added to such electrolytes, the composition of which affects the speed of creation and uniformity of the protective film. Copper passivation is carried out in solutions containing small amounts of sulfuric acid. When processing aluminum, phosphoric acid is included in the electrolytes, and additives in the form of nitric and sulfuric acids are used to passivate zinc and cadmium. The content of passivating solutions for processing steel products depends on their composition and often includes nitric acid and its salts.
All chromium salts (especially hexavalent) are very toxic. Therefore, chrome passivation of metal products can only be carried out in specialized industries that have appropriate cleaning and drainage systems, as well as specially trained personnel.
Nowhere is it written how passivation with chromium salts is carried out directly at the installation sites of the equipment. How are chemicals removed in these cases? Or are other compounds used for this treatment? If anyone has information on this issue, please share it in the comments to our article.
Passivation of structural and special steels
For reliable passivation of steels, it is advisable to pre-coat them, all or partially (those elements that will experience the greatest impact of adverse factors) with nickel, zinc or cadmium using chromium salts. Passivation with these salts is advantageous in that, after strengthening the surface layer, the products can be used for a very long time without the risk of corrosion. And if individual sections begin to rust, they can be passivated, without disassembling or removing the structure, with the same composition with chromium salts right on the spot, by applying overlays soaked in solutions.
What is passivation?
The passivation process allows stainless steel to return to its original properties, further protecting it from the effects of many external factors. This is a special chemical treatment of metal products, after which a special protective coating is formed on their surface. When interacting with concentrated acids, an inconspicuous film appears on stainless steel. This process is called passivation.
This method is used both for additional processing during the production of products and for restoring the basic properties of stainless steel parts.
History of the discovery of aluminum
For a long time, man has known the compound of the metal in question - potassium alum. It was used as a means that could swell and bind together the components of the mixture; this was also necessary in the manufacture of leather products. The existence of aluminum oxide in its pure form became known in the 18th century, in its second half. However, no pure substance was obtained.
Read also: Star-delta motor connection diagram
The scientist H. K. Ørsted was the first to isolate the metal from its chloride. It was he who treated the salt with potassium amalgam and isolated gray powder from the mixture, which was aluminum in its pure form.
Then it became clear that the chemical properties of aluminum are manifested in its high activity and strong reducing ability. Therefore, no one else worked with him for a long time.
However, in 1854, the Frenchman Deville was able to obtain metal ingots by electrolysis of the melt. This method is still relevant today. Especially mass production of valuable material began in the 20th century, when the problems of generating large amounts of electricity in enterprises were solved.
Today, this metal is one of the most popular and used in construction and the household industry.
Why is this necessary?
A stainless steel sheet has a very thin oxide film on its surface. It is this that prevents the formation of rust on parts, fasteners, and hardware made from this material. But the slightest violation of the integrity of this coating leads to the fact that the main anti-corrosion properties of stainless steel are lost. The causes of damage to the oxide film can be very different:
when the material comes into contact with chlorine; when steel interacts with sea water; in case of mechanical or physical damage, including scratches and minor dents.
Therefore, it is important to comply with the operating conditions that are regulated by the manufacturing plants of certain products (cutlery, fasteners, hardware, working tools, solid sheets, etc.). It is prohibited to use detergents containing chlorine and other aggressive chemicals.
But the greatest damage to the oxide film is caused by welding. This is especially harmful in the case of pipe welding. In such a situation, the protective surface is destroyed along the entire seam. Steel passivation is used to restore surfaces and protect products from rust. But here the composition of the stainless steel plays an equally important role.
Purposes of passivation of stainless steel welds
To passivate stainless steel and the seams formed on it after welding, solutions of various metals are used. As a result, phase layers are created that have new properties. The layers are more resistant to oxidation processes and help protect steel from the destructive effects of corrosion. The use of such processing allows you to achieve the following goals:
- The processes of destruction of the top layer of metal stop;
- The outer layer of the part is evenly smoothed, scratches and burrs are removed;
- The created welded joints are thus protected from loss of strength;
- In some cases, after processing, the metal becomes more elastic and its fragility decreases;
- Creating a protective film allows you not to fear the destructive effects of corrosion in the future;
- A layer is applied that improves the decorative properties of the object and improves its consumer qualities;
- The service life of products is significantly increased.
Carrying out passivation of welds is especially important for difficult-to-weld metals: this procedure helps ensure the tightness of the joints. The use of passivation is important because even air can be considered an aggressive medium.
Passivation of stainless steel is especially often used to protect pipe structures, fastening parts, and structural elements that must constantly come into contact with sea water.
However, when performing passivation, it must be taken into account that it is not desirable in all cases. Sometimes it has a negative effect on the strength of steel. Therefore, when deciding to carry out passivation, it is necessary to take into account all the features of the material being processed and the passivation method used.
Stainless steel classification
The anti-corrosion properties of stainless steel directly depend on its composition. Based on this, this steel is marked. The classification allows you to distinguish each type of stainless metal by flexibility, hardness, and degree of anti-corrosion protection. Depending on the composition and purpose, they are distinguished:
martensitic steels. Knives (including those for the food industry) and turbines are usually made from them. This steel, having a large percentage of chromium in its content, is very hard; ferritic materials. The amount of chromium in such steel exceeds the previous value by 3-4%. This material has high resistance to phosphoric acid, ammonium nitrate and nitric acid; austenitic steels. This type of stainless steel is very ductile. It is often used in mechanical engineering; duplex or ferro-austenitic metals. These are very durable, but at the same time flexible stainless materials.
Based on the composition of the stainless steel, you can determine whether there is a need for additional processing of the products or not. The likelihood of corrosion on the surface of elements made from this type of steel also depends on this.
Causes of corrosion
Despite the fact that the chemical composition of stainless steel must contain passivators that significantly increase its corrosion resistance, its surface and internal structure can be subject to corrosion.
The main reason why stainless steel begins to deteriorate is insufficient or uneven chromium content in its chemical composition. Contact with metal, which is significantly less resistant to oxidation, can also cause corrosion. Stainless steel products that were connected to each other using welding technology are often subject to destruction.
Corrosion of heated towel rail pipes caused by dishonest welding by the manufacturer
Technology and methods
There are various methods for processing stainless steel. But there are two main methods of steel passivation:
Etching with chemical acids (concentrates) in certain areas. This technology is often used for processing welds, but is also allowed in other cases. This process has different processing sequence options. They differ both in the composition of chemicals and in the time of work. The most common method in this case is electrolytic etching. This technology consists of placing a stainless steel product in a specially prepared bath consisting of concentrated acids. An electric current (alternating or direct) is passed through this composition. The metal plays the role of either a cathode or anode. The supplied current has a mechanical effect on the steel, resulting in the release of hydrogen or oxygen gas. This helps to separate the oxide film on the surface of the product. Etching with ready-made acid mixtures. They can be made in the form of pastes, gels, sprays, concentrates. This method is the most convenient.
Regardless of which method is used to passivate stainless steel, it is important to follow the sequence of work.
Stainless steel and rust. Passivation
We all know about the existence of stainless steel, but not everyone knows its composition. Thus, ordinary steel consists of iron alloyed with carbon, and its percentage usually does not exceed one. As for stainless steel, in addition to iron, chromium can be found in its composition. It accounts for approximately 10 to 30%.
Chromium is the very element that protects stainless steel from corrosion. How does this happen? Chromium reacts with oxygen, thus creating a protective layer on the surface (chromium oxide). It is this that protects the product from corrosion and rust.
But do not forget that the protective layer does not give a 100% result, because it can be damaged, which leads to a weakening of the protection, and, consequently, to corrosion with rust.
How can you destroy the protective layer? There are several ways:
1. Bleach (and other cleaning bleaches). With these materials you can completely destroy the protective layer;
2. Scratches;
3. Excessive cleaning;
4. Impact of steel jaws;
5. Contact with steel susceptible to rust. Ordinary iron can damage stainless steel due to its properties, thus destroying the protective layer;
Passivation of stainless steel to protect it
Many people are interested in how stainless steel products are made. In this article we will answer this question.
To begin with, it should be noted that at the end of production, the products are placed in nitric acid to get rid of various contaminants. This also promotes the oxidation of chromium in the air. This process is called passivation. It lasts about 20 minutes.
Of course, it is always possible to find cheaper equipment, but in this case there is a possibility that the product will not be completely cleaned and washed. Therefore, sometimes it is better to pay a slightly higher price, but be sure that stainless steel products, especially if you plan to use them in the food industry, have been fully processed, cleaned and protected.
Now let's talk in more detail about passivation at home:
1. Cleaning. First of all, the product undergoes scrupulous cleaning, especially if you do not have the opportunity to use nitric acid. But what to use? A strong cleaning agent (for example, sodium triphosphate) is suitable for this. It must be mixed with hot water. It is necessary to clean not only the surface of the product, but also small parts. After cleaning, rinse and dry the product.
2. Passivation using acid. After removing dirt, oils and various impurities, the next step of passivation begins. A weak acid is used for this. For example, citric acid. You should mix it with warm water. A 4-10% concentration is sufficient. It is necessary to keep the product in this solution for about 30 minutes. After this, the product must be left to air dry (preferably overnight) so that the chromium reacts with oxygen and forms a protective layer.
Important!
Do not forget about your safety when working with acids! Be sure to use gloves to protect your skin from irritation.
Stages of chemical passivation
In the process of forming a homogeneous inert film on the surface of stainless steel products, it is important to take into account the characteristics of the steel composition and the degree of damage to the protective coating. Chemical passivation today is an integral part of working with stainless materials. This allows you to extend their service life, get rid of rust and damage, and prevent the formation of corrosion. During passivation work, the following sequence of steps should be followed:
First, the materials are cleaned from contaminants. Grease stains, rust and other deposits are removed. With chemical acid etching technology, the product is immersed in a bath with a mixture of hydrochloric acid and sulfuric acid. At temperatures from 60 to 80 degrees, the steel is kept here for 20-40 minutes. If the method of etching with ready-made mixtures of acids is used, then special concentrated compositions (pastes, gels, sprays) are used for cleaning, which are applied to the surface of the steel manually. The chemical is left for approximately 30 minutes. Then the products are thoroughly washed with water. The passivation process begins. In the first case, the steel is immersed in an acid bath. In the second, gels, pastes, sprays and other ready-made chemical compositions are applied to the surface of the product. In the case of ready-made products, one more stage is provided - treatment with a passivator. This allows for the forced formation of an oxide film on stainless steel. The last stage consists of thoroughly washing the product.
The composition of stainless steel and the grade play an important role in the appearance of the product after chemical passivation. Some species are dark in color, while others are lighter. But regardless of this, this method of steel processing has a whole list of advantages:
improves resistance to corrosion; the surface of the product is uniformly smoothed; burrs, scratches, dents are removed; The service life of the products is significantly increased.
Passivation and care of stainless steel brewing equipment
Despite its reputation as an ideal metal for beer production, stainless steel can cause corrosion or rust. So this week we'll take a look at how and why stainless steel can corrode, as well as how you can passivate your stainless steel brewing equipment to protect it.
Stainless steel and rust
Steel is made from an alloy of iron and carbon, and carbon makes up only half or a little over a percent of its composition. In comparison, stainless steel is made from iron and chromium. Chromium contains approximately 10-30% of steel, and it is an important element that makes stainless steel resistant to corrosion.
The chromium in stainless steel reacts very quickly with oxygen, and actually forms a protective layer of chromium oxide on the surface of the steel. This chromium oxide prevents the formation of rust and corrosion. However, if the chromium layer is compromised for any reason, the iron in the steel can actually begin to corrode and rust.
Your stainless brewing equipment is generally very resistant to corrosion. However, if you expose it to bleach or other bleach cleaners, scratch it, over-clean it, or expose it to regular rusting steel pads or leave it in contact with regular steel, it can damage the protective layer. Bleaching agents can completely remove the protective layer.
Excessive cleaning, especially with steel wool, can also undermine your oxidation layer. It is important to store regular steel in the same place as regular buckets, tools and some equipment other than your stainless steel equipment. Iron from ordinary steel tends to damage stainless steel (a property of iron) and destroy the oxidation layer.
Do not place regular steel buckets or mixed metal tools or equipment in your stainless steel kettle after boiling.
Passivation of stainless steel to protect it
When stainless steel products are made, they are typically immersed in a nitric acid bath at the end of the manufacturing process to remove contaminants. The acid also activates the oxidation process of chromium in the air called passivation, where a protective layer of chromium oxide is formed when oxygen reacts with chromium. Passivation occurs very quickly - usually within 20 minutes.
Now some stainless brewing equipment, particularly lower cost stainless materials, were likely machined, stamped, pickled, polished and welded only after the stainless steel had been fabricated and acid washed. As a result, it may contain oils, polishes, welding compounds and other contaminants that protect the steel but should be washed away the first time you clean your parts. Plus, you probably don't want to find these oils and compounds in your beer.
Step 1: Thorough Cleaning
Since you likely don't have access to a large bath of nitric acid, at-home passivation begins with a very thorough cleaning. If this is new equipment where you want to remove any substances left over from manufacturing and finishing. This will require a strong cleaning agent such as trisodium phosphate (TSP). Mix TSP in the recommended proportion with hot water.
Bar Keeper's Friend is also a good cleaner for stainless steel equipment (Note: it is an imported version similar to Pemolux powder but more effective), although you should not use it on etched metals. They also sell a "soft scrub," a liquid version of Bar Keeper's Friend that is easier to use. Make sure you remove all fittings, valves and other small items and clean them too.
Rinse and dry everything thoroughly after cleaning.
Step 2: Acid passivation
Now that all dirt, oils and impurities have been removed, you can begin the next step of metal passivation. This is achieved by using a weak acid and then air drying the metal. Oxygen contained in the air will interact with chromium, forming a passive protective layer. Always wear gloves when working with these acids as they can cause skin irritation in high concentrations.
There are several options you can use here. One option is to use Bar Keeper's Friend, which contains oxalic acid. It works well on stainless steel, but do not use it if you have had electronic etching applied to the surface of your equipment as it will destroy or even remove the etching.
Add enough water to form a thick paste and apply the product to the object that requires passivation. Let it “sit” on the metal for 5-10 minutes, and then gently wipe it with a dry towel.
Essence of the process
Passivation is not an electrolytic finishing operation that increases the corrosion resistance of stainless steels. The passivation process typically uses dilute nitric or citric acid to promote the formation of an inert protective oxide layer. It is more inert to air, so it slows down subsequent corrosion.
The acid chemically removes—dissolves—free iron from the stainless steel surface, replacing it with a thin surface film of less reactive oxides. Since any stainless steel contains a large amount of chromium, passivation results in the formation of chromium oxide, which has an increased thickness. The surface is passivated and rust protection is improved. At the same time, surface contaminants are removed.
First cleaning
- Grease, coolant or other contaminants must be thoroughly removed from the surface to obtain the best corrosion resistance. A commercial degreaser or detergent can be used to clean mechanical oils or coolants. Foreign matter such as thermal oxides may need to be removed by grinding or by methods such as acid etching.
- Sometimes the operator may skip basic cleaning, incorrectly assuming that simply immersing the grease in an acid bath will both clean and passivate simultaneously. This doesn't happen. Instead, the grease contaminant reacts with the acid to form gas bubbles. These bubbles collect on the surface of the workpiece and interfere with passivation.
- Even worse, contamination of the passivation solution, sometimes with high chloride content, can cause a corrosion “flare-up”. Instead of producing the desired oxide film with a shiny, clean, corrosion-resistant surface, flash causes a heavily etched or darkened surface—the deterioration of the surface itself that passivation is designed to optimize.
- Parts made from martensitic stainless steels [which are magnetic, moderately resistant to corrosion, and have a yield strength of up to (1930 MPa)] per square inch are hardened at high temperature and then annealed to provide the required hardness and mechanical properties. Precipitation hardenable alloys (which provide a better combination of strength and corrosion resistance than martensitic grades) can be solution processed, partially processed, held at lower temperatures, and then finished with machining.
- In such cases, the parts must be thoroughly cleaned with a degreaser or cleaner to remove traces of cutting fluid before heat treatment. Otherwise, the cutting fluid remaining on the parts will cause excessive oxidation. This condition can cause the underlying layers to remain mottled even after descaling by acid or abrasive methods. Cutting fluids may remain on the parts and harden in a vacuum oven or protective atmosphere, and carburization of the surface may occur, resulting in loss of corrosion resistance.
Etching
- Pickling is the removal of the adjacent low chromium metal layer from the surface of stainless steel by chemical means.
- Where steel is heated by welding, heat treatment or other means to the point that a colored oxide layer can be seen, there is a chromium-depleted layer on the surface of the steel below the oxide layer. Lower chromium content gives lower corrosion resistance. To restore the best corrosion resistance, the damaged metal layer must be removed, exposing the fully alloyed stainless steel surface. Mechanical removal may produce abrasive or other particles (corrosion inhibitors) or may be impractical, so chemicals are usually used.
- Procedures involving etching solutions of nitric (HNO3) and hydrofluoric (HF) acids remove the scale and chromium-depleted underlayer and restore corrosion resistance. Etching solutions also remove contaminants such as iron and iron particles. Etching solutions other than mixtures of nitric and hydrofluoric acids exist and can be used for specialized applications.
- Etching pastes, where a solution is mixed with an inert carrier, are typically used to treat selected areas such as welds.
- Etching involves the removal of metal and a change in the visual brightness of the metal.
- Electropolishing is a useful alternative to etching. Metal removal is achieved but usually results in a bright, smooth and more corrosion resistant surface.
What oxidizing agents are required for passivation?
The main condition for passivation of stainless steel is that passivation does not destroy the base metal. Therefore, the oxidizing agent must be “soft”, with a relatively low pH. Under such conditions, a protective passive film forms spontaneously. It is better to use citric acid as such substances, since organic acids work more gently than mineral ones, and they do not require special preparation.
Is it possible to do without passivation? Stainless steel has corrosion-resistant properties due to its chromium content, but is not completely impervious to corrosion. Oxidizing in the presence of citric acid, chromium forms a moisture-resistant surface film.
Aluminum passivation
On aluminum, an oxide and very durable film is formed under natural conditions under the influence of atmospheric oxygen. Many people remember the school experience when a small layer was removed from an aluminum wire dipped in mercury using a file, and then this file-treated tip was removed from the mercury. And the treated end in air was instantly covered with a “fur coat” of oxide crystals. But under normal atmospheric conditions, oxides do not form on aluminum so quickly and take the form of a transparent film with a thickness of only a few microns. In its properties it is very close to the chemically inert aluminum oxide corundum. The disadvantage of such a natural film is its instability with a significant increase in temperature or with prolonged exposure to active acids.
For lasting protection, one cannot do without the anodizing process, which results in protective films with a thickness of 5 to 20 microns. And in certain modes it is possible to obtain ultra-strong films (withstanding loads of up to 1500 kg per mm, that is, higher than that of tool steel.
Sequence of passivation
The following procedure for carrying out the technology under consideration is recommended:
- Preliminary cleaning of the surface of the part to be passivated from any contamination.
- Chemical treatment by immersing the material in a bath of citric acid.
- Washing in water.
- Neutralization of acid residues in an aqueous solution of sodium carbonate.
- Drying.
- Testing the finished surface (the electrical contact measurement method is used, since the conductivity of the passivated layer is worse than that of a conventional one).
Passivation is recommended for all grades of stainless steel that contain more than 0.02% sulfur (even if the surface visually appears clean and shiny). Particularly desirable is the processing of steels containing sulfides, as well as titanium and tantalum - metals whose oxides are relatively quickly destroyed in a humid atmosphere.
To enhance the efficiency of passivation, sodium dichromate is usually added to acid bath solutions. Options with the simultaneous application of ultrasonic vibrations are more productive: under such conditions, the formation of chromium oxide is intensified, which begins even when the material being processed is in an acid bath.
The thickness of the passivating film is very small - up to 5 microns, but this is enough to reliably protect the stainless steel surface from corrosion.
Etching process
Before etching, it is necessary to thoroughly clean and degrease the metal surface from foreign substances such as grease, oil, adhesives, rust, etc. Surface cleaning can be done with any cleaner, including alkaline cleaners, acidic cleaners, and solvent-based cleaners. The correct cleaning solution is selected based on several factors:
Material and configuration of equipment/parts Level and composition of pollutants
After cleaning and degreasing, the cleaning solution is washed off the surface and etching is carried out using one of the methods mentioned above. Process control is very important as corrosion and pitting can occur if the acid concentration is too high and/or if the acid contact time is too long. Once the process is complete, be sure to ensure that all residual acids are removed and neutralized to prevent pitting and pitting. To achieve optimal corrosion resistance, it is recommended to passivate the stainless steel surface.
Chemical passivation as an optimal coating for heat-resistant steel
Metal passivation is a process as a result of which an oxide film is formed on the surface of the metal, preventing the formation of corrosion. The name of the coating method comes from the word “passivity”. The purpose of passivation is to reduce the chemical reactivity of a metal when interacting with other metals or aggressive environmental conditions.
In its own way, the appearance of a film is the same destruction of metal. But by destroying the top layer of material by several tens of nanometers, passivation saves the lower layers from rust.
Thus, chemical passivation is the interaction of an oxidizing agent with the surface being treated.
Stages of chemical passivation
1. If you do not prepare the metal product first, the oxidizing agent will react not with the alloy, but with foreign elements. Therefore, before passivation it is necessary to clean the surface . Cleaning is carried out in 2 ways: by washing or sanding the product using sandpaper. Now you can start passivation.
2. The process itself involves applying a chemical reagent to the product . A protective film is formed on the alloy, consisting mainly of salts and oxides. The film makes the structure of the product stronger and more durable. The effectiveness of the procedure depends on the following factors:
- composition of the solution;
- alloy composition;
- condition of the surface of the workpiece.
High-alloy steels, especially chromium-nickel steels, are best suited to chemical passivation. But carbon steels should be treated only for short-term protection, since the level of the protective layer on them is significantly weaker.
3. Cleaning with water . Any salts that may remain on the product may cause corrosion. Therefore, washing should be carried out carefully.
4. Residual acid must be neutralized with a 2-3% ammonia solution or a solution consisting of 25-30 g/l oleic acid and 2-4 g/l sodium hydroxide. Treatment is carried out at 80 – 90 °C for 2-3 minutes.
What solution is used?
The use of different solutions depends on the properties of the alloy. Let's consider what solutions are used to passivate various classes of ferrous metals:
Highly alloyed alloys resistant to corrosion - nitric and sulfuric acids.
- Ferritic alloys - potassium dichromate, nitric acid.
- Carbon steels - potassium dichromate, chromic anhydride, phosphoric acid, sodium hydroxide.
- Medium alloy steels - chromic anhydride, phosphoric acid.
The passivation temperature and time also depend on the alloy class. The temperature ranges from 18 to 90 °C, and the time ranges from 3 to 60 minutes.
The higher the temperature of the solution, the faster the process proceeds.
Application of passivation
- Passivation is used for metal parts for painting. It not only protects against corrosion, but also degreases products. Used in the field of mechanical engineering.
- Passivation of steam turbines. But why do you need passivation of stainless steel, since it won’t rust anyway? It turns out that if the alloy is in continuous contact with an aggressive environment, it can collapse. An example is a weld. Sometimes there are iron particles on it. And then even stainless steel corrodes.
- Dental field. The lower part of the implants is processed - the screws that are mounted into the jaw. Passivation is used to prevent destruction of the implant in the jawbone.
- Chemical passivation is often carried out for decorative purposes. With short-term treatment, a rainbow film appears on the surface. Bright objects of use - taps, door handles.
- Passivation of costume jewelry is used to avoid allergic reactions.
Chemical passivation significantly extends the service life of metal products and deserves wide application in a wide variety of fields.
Passivation of surfaces
Almost all metals are quite durable materials. However, their structure and general condition can be affected by ordinary oxygen or liquid. Under the influence of an aggressive environment, plaque accumulates on the surface of metal products, which is corrosion. It is dangerous because under its influence the structure of the metal is destroyed, and the product made from it becomes unsuitable for further use.
Passivation has found widespread use in the modern world. It is not an easy procedure. It is almost impossible to cope with this without certain knowledge. The procedure is to dissolve the top part of the metal using an anode. In this case, the molecules break down into substances that have different levels of charge. In order for the ions to acquire an ordered appearance, an electric current is applied to the metal at a low voltage level, which is only 6-12 volts.
Ions are divided into positively charged and negatively charged. When an electric current passes through a metal, positively charged particles tend to the cathode, and negatively charged particles tend to the anode. It is at the anode that metal oxides are formed, which are the result of the splitting of the upper metal layer. As a result, a very thin protective film appears on the surface of the processed metal, which has unique protective qualities.
Passivation is aimed at making the metal less active. It becomes passive and is practically not affected by the environment.
In modern industries, this procedure is quite in demand. It helps protect metal surfaces from corrosion. The passivation process is used in situations where there is a need for careful preparation of the surface for applying paint and varnish. Also, this procedure is indispensable in those enterprises where metal objects often have to interact with an aggressive environment.
Passivation of metals is a useful procedure that renders these substances passive. It allows them to retain their properties for a long time. The thin film has an excellent level of protection, which gives metals additional strength and hardness.
Passivation of silver
Silver is a noble metal, despite changes in its properties in the light (it darkens). Before the advent of digital photography, this ability of silver was used in the creation of light-sensitive materials (film and photographic paper).
But the darkening of silver products in everyday life is often an undesirable process, and to prevent it, chemical methods are used to protect the upper layer of metal, bordering with air, from exposure to light and air. The best way to prevent such changes is passivation by treating silver in a chromium peak - potassium dichromate K2 Cr2 O7.
To carry it out, 60 g of chrompic is diluted in 1 liter of boiled, soft water. The working temperature of the solution is from 25 to 40 degrees, this is not critical. Passivation is carried out by simply immersing the silver item in the bath completely for 20 minutes and periodically stirring the solution. In cases where the diluted amount of chromium does not completely cover the product (a figurine of a complex shape or a voluminous silver candelabra), it is better not to practice alternating surface treatment in parts, but to dilute the reagent in the amount of water required for the normal volume.
Properties of metal after processing
The main objective of passivation is to improve the physicochemical and mechanical characteristics of the surface layer of the material from which the part is made. The remaining characteristics of the deeper layers remain unchanged. Therefore, after passivation is completed, the following properties and characteristics change in the surface layer:
- a layer with a new chemical composition appears;
- anti-corrosion activity changes (it slows down significantly);
- the physical characteristics of the material are improved (only the surface layer);
- in some cases, the mechanical strength of the product increases;
- the color of the part changes (it takes on a more aesthetic shape);
- consumer properties increase and presentation improves.
Passivation of stainless steel can significantly improve anti-corrosion properties and give the finished part a completely different color. The use of chromium or nickel in the passivation solution produces a shiny metallic color.
Passivation of iron with chemical elements close to it makes it possible to create an outer layer that is sufficiently resistant to corrosion. In this way, the scope of application of such products expands. They can be used even in active and aggressive environments. In addition to various grades of steel, cast iron is subjected to passivation. The main task is to create a protective film against corrosion. In some cases, when thickened sodium nitrate is used, the surface layer acquires some elasticity. In this case, the fragility of the entire part is reduced. One type of steel is the so-called blued finish. The result of processing is a reliable outer layer of black color.
The properties of the surface layer of non-ferrous metals change in a similar way. As a result of passivation, adsorption or phase layers of a certain thickness are formed. Placing an aluminum workpiece stimulates the process of natural passivation of the surface layer of this metal. When exposed to acidic solutions, the protective properties of the surface layer of aluminum increase.
Passivation of steel
Iron, which is part of any type of steel, as its basis, is subject to corrosion more than any other metal. The best protection against corrosion for iron-containing materials is the addition of alloying additives to the iron melt, which make the steel stainless. But stainless steel is expensive. Therefore, simpler grades of steel can be protected from rust by treating them in electrolytic baths with the addition of inhibitory pigments in the form of red lead - iron or lead - to the electrolyte.
| These pigments can also work as chemical passivators, without the use of a complex mechanism for connecting them with the metal being coated. The application of such pigments is carried out with ordinary painting supplies, and is usually associated with the large dimensions of the treated surfaces, which cannot be placed in an electrolytic bath (hulls of all types of ships). But in this case the protective effect will be weaker. |
During anodic coating with the help of pigments in the boundary treated outer layer, a high current density occurs in the pores of the formed protective film. In iron, as part of a steel alloy, protective oxide films cannot be formed under natural conditions, so passivation is possible only if inhibitor pigments are included in the coating mechanism.
But the main difference in the formation of protective layers on metal by chemical and electrolytic passivation methods lies in the speed of the process and the strength of the formed phase film. After all, both in a chemical bath and in it, but with electric current and voltage added to the process, the process of formation of an oxide or salt film follows the same scenario.
Principles of cleansing at home
Tips for caring for stainless steel are very simple. They are easy to follow.
What not to use
List of products and devices that should not be used when using stainless steel cookware:
- Dishwasher;
- metal sponge;
- a cleaner containing abrasive components.
How to use baking soda and salt
Salt and soda are essential care products for stainless steel cookware. The principle of their use is simple:
- the product is washed;
- apply soda, salt or a mixture thereof to the contaminated area;
- rub the powder in a circular motion.
After cleaning, the item is rinsed with water and dried with a towel.
Timeliness
Regular cleaning of stainless steel pans eliminates the appearance of old stains. It takes little time to remove fresh stains.