The industry uses natural graphite, as well as artificial varieties of this material. Its wide range of applications is due to its unique physical and chemical properties, which may differ slightly for different brands and types of graphite.
IMPORTANT PHYSICAL AND CHEMICAL INDICATORS OF GRAPHITE:
- Thermal conductivity;
- Electrical conductivity;
- Expansion due to heat;
- Strength;
- Solubility;
- Wettability;
- Anisotropy of properties.
THERMAL CONDUCTIVITY OF GRAPHITE:
- Natural types of this material are characterized by high thermal conductivity (many metals are inferior to graphite in this indicator). It is influenced by the final processing temperature of a particular grade of graphite, but the average is 3.55 W*degrees/cm, and the thermal conductivity coefficient is 0.041. It should be noted that thin graphite filaments conduct heat better than copper counterparts.
- At a pressure of 0.9-1 atmosphere, graphite boils, reaching a temperature of 4200 degrees, and melts at 3845-3890 degrees.
- Crystalline varieties of the material ignite in an oxygen stream at 700-730 degrees Celsius.
- Combustion of graphite releases a sufficient amount of heat - up to 7856 kcal.
ELECTRICAL CONDUCTIVITY OF MINERAL
Graphite is an excellent conductor of electricity - in this indicator it is superior, for example, to mercury. Heating the mineral helps improve the conductivity of electrical current. Thus, the mineral has a negative temperature coefficient of resistance. At 0 degrees it is in the range of 0.39-0.602 ohms. As for the resistivity limit, it is the same for all types of material and is 0.0075 Ohm. These properties explain the widespread use of graphite in electrometallurgy.
Receiving technologies
Almost all graphite products undergo processing operations before final use. The method of production also determines the type of graphite material. As a rule, the difference in methods is determined by the temperature effect. Thus, by heating a mixture of pitch and coke, Acheson graphite is obtained. The melting and boiling points in this case will be 2800 and 4200 °C, respectively. The thermomechanical method of processing the coke mixture involves exposure to the same heating rates - the only difference is the use of carbide-forming components. The pyrolysis method is characterized by low temperature treatment rates. In this case, natural graphite is modified from gaseous hydrocarbons in a vacuum at 1500 °C. At the same time, cooling methods for processing base mixtures to produce graphite are also common. These technologies include blast furnace, during which the cast iron masses are slowly cooled.
STRENGTH
Another characteristic that sets graphite apart from other natural minerals. It changes depending on the temperature. For most brands (including artificial types of material), when heated, the tensile strength in bending, compression and tension increases (up to two times). The maximum is achieved at 2200-2800 degrees. If the temperature rises above 3000 degrees, the strength characteristics rapidly drop.
Recrystallized material has the highest strength.
Place of Birth
There are several graffito-bearing provinces: Ukrainian, Ural, Tunguska (Noginskoye, Kureyskoye), Verkhne-Sayan (Botogolskoye), Ussuriysk and others.
Large deposits of graphite are found in South Korea, Mexico (Sonora state), Malagasy Republic, Sri Lanka, India, Germany and Sweden.
The main method of enriching cryptocrystalline ores is ore sorting, while densely crystalline and flake ores are flotation. The quality of concentrates is subject to restrictions on ash content and particle size distribution (graphite flakes are valued by size). Cryptocrystalline ores are ground.
When flotation of flaky and densely crystalline ores, collectors are used - kerosene and other hydrocarbons; foaming agents - pine oil, alcohol; regulators - soda, alkali; depressants - starch, dextrin-based reagents. To improve selection, liquid glass is supplied.
Flotation is followed by wet classification, drying, air classification and hydrometallurgical operations, including sintering with soda, boiling the cinder, leaching with sulfuric acid, washing, boiling in soda solution, washing, drying and dry magnetic separation to obtain graphite in a non-magnetic product. When finishing flake blast furnace graphite, electrical separation is used.
ANISOTROPY OF PROPERTIES
Since the characteristics of different grades of graphite differ, they are influenced by the method of pressing, for artificial types - the orientation of the coke grains.
Graphite is quite plastic and easy to machine. Different types differ in the level of fat content, which is why it is used as a lubricant. It is necessary to note one more feature of pure types of this material: graphite has the highest moderation coefficient and low neutron absorption rate.
Characteristics of graphite
Graphite is a representative of the class of native elements of high strength. Its structure has a large number of layers.
There are two types of graphite found in nature:
- coarse crystalline,
- fine-crystalline.
Based on the size of the crystals and their location relative to each other, the following types of graphite are found in nature:
- clearly crystalline,
- cryptocrystalline.
Graphite has a fairly layered structure. Each layer has a wavy shape. It is mild.
Graphite is one of the elements that consists mainly of crystals of different sizes. They have a plastic structure and small scales along the edges. In terms of their strength, they can be compared to diamonds.
The crystal lattice of graphite consists of a large number of layers, which have different locations relative to each other.
Today, artificial graphite is often produced, which is created from a mixture of various substances. It is used in various sectors of human life. Artificially produced graphite has a large number of types.
In the modern world, it is planned to extract gold from graphite. Scientists have found that one ton of graphite contains approximately 18 grams of gold. This amount of gold ore is inherent in gold deposits. Currently, it is possible to obtain gold from graphite not only in our country, but also in other countries of the world.
APPLICATION
For the manufacture of melting crucibles, lining plates - the application is based on the high temperature resistance of graphite (in the absence of oxygen), on its chemical resistance to a number of molten metals. It is used in electrodes and heating elements due to its high electrical conductivity and chemical resistance to almost any aggressive aqueous solutions (much higher than that of noble metals). For the production of chemically active metals by electrolysis of molten compounds, solid lubricants, in combined liquid and paste lubricants, plastic filler.
It is a neutron moderator in nuclear reactors, a component of the composition for the manufacture of leads for black graphite pencils (mixed with kaolin). Used to produce synthetic diamonds, as a nanometer length standard for calibrating scanners of a scanning tunneling microscope and atomic force microscope, for the manufacture of contact brushes and current collectors for a variety of electrical machines, electric vehicles and overhead cranes with trolley power, powerful rheostats, as well as others devices that require reliable moving electrical contact for the manufacture of thermal protection for the nose of ballistic missile warheads and re-entry spacecraft.
Graphite – C
| Molecular weight | 12.01 g/mol |
| origin of name | from ancient Greek γράφω - to record, to write |
| IMA status | valid, first described before 1959 (before IMA) |
Therapeutic effect
Homeopaths were the first to appreciate graphite. They found that the mineral is suitable for the treatment of skin pathologies (eczema, psoriasis, lichen, etc.).
Today the list has been expanded:
- Metabolic disease.
- Malfunction of the thyroid gland.
- Respiratory tract diseases (rhinitis, bronchial asthma).
- Gastrointestinal problems (gastritis, gastric ulcer, duodenal ulcer, colitis).
- Women's ailments (amenorrhea, chronic inflammation of the ovaries, mastopathy).
- Conjunctivitis, cataracts, stye.
The mineral also “supervises” emotional health. It is prescribed for morning headaches, neurasthenia, apathy, and depression.
Where and how is it mined?
There are commercial-scale graphite deposits on all continents:
- Both Americas - USA, Canada, Brazil;
- Europe – Germany, Greenland, Italy;
- Australia.
The raw materials of each graphite mine can be distinguished by structure, color, and other characteristics.
Russia has three largest deposits:
- Buryatia – high-quality densely crystalline raw materials.
- Krasnodar region (two) – dense, finely crystalline, scaly, graphite shales.
Graphites are formed by coal pyrolysis or under the influence of extremely high temperatures and pressure. For example, the outpouring of magma onto coal deposits.
It is mined by above-ground or underground methods. Graphite crystals are found in shales, marbles, and other organic rocks.
The annual global production of graphite is 600 thousand tons.
MORPHOLOGY
Well-formed crystals are rare. The crystals are lamellar, scaly, curved, and usually have an imperfect lamellar shape. More often it is represented by leaves without crystallographic outlines and their aggregates. Forms continuous cryptocrystalline, foliated or rounded radial-radiating aggregates, less often – spherulitic aggregates of a concentric-zonal structure. Coarse-crystalline precipitates often exhibit triangular shading on the (0001) planes.
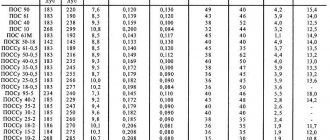
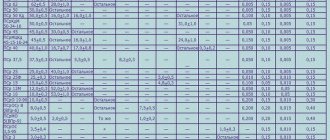
Main brands
Today, the possibility of synthesis with different grain sizes is used. As a result, graphite can be classified into:
- coarse-grained;
- medium grain;
- fine-grained;
- fine-grained.
The elements of the first reach a diameter of 3,000 microns. If we are talking about a medium-grained variety, then the grain size is 500 microns. There is a variety of fine-grained graphite grade MPG with a grain size of up to 50 microns. There is also a fine-grained isotropic mineral of the MIG-1 brand, the particles of which have sizes from 30 to 150 microns. Fine-grained graphite and isostatic graphite have grains up to 30 microns in size, their minimum diameter is 1 micron.
Graphite
Home / Cases / Graphite
Practical meaning:
Graphite is used very widely. We can say that there is not a single industry where it is not used to one degree or another. Graphite is needed mainly in the metallurgical industry for the manufacture of refractory crucibles and for coating the surface of foundry molds in order to protect the casting from burning of the molding earth; in addition, in the electrical industry - in the production of electrodes and arc carbons, in the production of pencils, black paints, black copy paper, printing ink or Chinese ink. It is also used as a lubricant (in cases where oil cannot be used due to high heat) and in steam boilers as an anti-scale agent. Recently it has been used for the manufacture of graphite blocks for “nuclear boilers” and for the manufacture of space technology. Artificial diamond is obtained from graphite. Graphite liquid is used for volumetric pressing of automobile parts. Dies coated with this substance ensure high surface cleanliness of steel workpieces, which eliminates their subsequent wiping on grinding machines.
Properties:
Conducts electricity well. Unlike diamond, it has low hardness (1 on the Mohs scale). Relatively soft. After exposure to high temperatures it becomes slightly harder and becomes very brittle. Density 2.08-2.23 g/cm³. The color is dark gray, metallic luster. Infusible, stable when heated in the absence of air. Greasy (slippery) to the touch. Natural graphite contains 10-12% admixtures of clays and iron oxides. When rubbed, it separates into separate flakes (this property is used in pencils).
Information
The industry uses natural graphite, as well as artificial varieties of this material. Its wide range of applications is due to its unique physical and chemical properties, which may differ slightly for different brands and types of graphite.
IMPORTANT PHYSICAL AND CHEMICAL INDICATORS OF GRAPHITE:
- Thermal conductivity;
- Electrical conductivity;
- Expansion due to heat;
- Strength;
- Solubility;
- Wettability;
- Anisotropy of properties.
THERMAL CONDUCTIVITY OF GRAPHITE:
- Natural types of this material are characterized by high thermal conductivity (many metals are inferior to graphite in this indicator). It is influenced by the final processing temperature of a particular grade of graphite, but the average is 3.55 W*degrees/cm, and the thermal conductivity coefficient is 0.041. It should be noted that thin graphite filaments conduct heat better than copper counterparts.
- At a pressure of 0.9-1 atmosphere, graphite boils, reaching a temperature of 4200 degrees, and melts at 3845-3890 degrees.
- Crystalline varieties of the material ignite in an oxygen stream at 700-730 degrees Celsius.
- Combustion of graphite releases a sufficient amount of heat - up to 7856 kcal.