- Characteristics
- Areas of application
- Operating rules
- Anti-rust application
- Application for metal
Often metal and products made from it are subject to a characteristic “disease”, which manifests itself in the form of a red coating that corrodes the metal. We're talking about rust. Its formation occurs due to the effect of carbon dioxide, oxygen and water on the surface of a metal product. Of course, in order to extend the life of a metal product, it is necessary to begin the fight against corrosion as soon as possible. Treatment with phosphoric acid can help with this.
What is phosphoric acid
At room temperature these are hygroscopic, colorless, diamond-shaped crystals that are highly soluble in water. An orthophosphoric compound is considered an inorganic acid of medium strength. One of its forms is a yellowish or colorless syrupy liquid, odorless, an aqueous solution with a concentration of 85%. Its other name is white phosphoric acid.
The chemical orthophosphorus compound has the following properties:
- soluble in ethanol, water, solvents;
- forms 3 rows of salts - phosphates;
- causes burns upon contact with skin;
- when interacting with metals, it forms flammable, explosive hydrogen;
- the boiling point depends on the concentration - from 103 to 380 degrees;
- the liquid form is prone to hypothermia;
- incompatible with flammable materials, pure metals, quicklime, alcohol, calcium carbide, chlorates;
- at a temperature of 42.35 degrees it melts, but does not decompose.
About caustic and not so caustic
– These idiots placed a porcelain container with “jelly” in a special chamber, extremely isolated... That is, they thought that the chamber was extremely isolated, but when they opened the container with manipulators, the “jelly” went through the metal and plastic, like water through a blotter, and escaped outward, and everything he came into contact with again turned into “jelly.” Thirty-five people were killed, more than a hundred were maimed, and the entire laboratory building was completely unusable. Have you ever been there? Magnificent building! And now the “jelly” has flowed into the basements and lower floors... Here is the prelude to contact.
— A. Strugatsky, B. Strugatsky “Roadside Picnic”
Hello, %username%!
Blame this person for the fact that I’m still writing something. He gave me the idea.
Just after some thought, I decided that a short excursion into caustic substances would be relatively quick. Maybe someone will be interested. And for some it’s useful.
Go. Let’s immediately define the concepts.
Corrosive - 1. Chemically corrosive. 2. Sharp, causing irritation, pain. 3. Sargent, caustic.
Ozhegov S.I.
Dictionary of the Russian language. - M.: Rus.yaz., 1990. - 921 p. So, we immediately discard the last two meanings of the word. We also discard “caustic” lachrymators - which are not so much caustic as they cause lacrimation, and sternites - which cause coughing. Yes, below there will be substances that have these properties, but they are what is important! - really corrode materials, and sometimes flesh.
We will not consider substances that are caustic only for humans and the like - due to the specific destruction of cell membranes. Therefore, mustard gases will remain out of use.
We will consider compounds that are liquids at room conditions. Therefore, we will not consider liquid oxygen and nitrogen, as well as gases such as fluorine, although they can be considered caustic, yes.
As always, the view will be purely subjective, based on personal experience. And yes - it’s quite possible that I won’t remember someone - write comments, %username%, within three days from the date of publication I will supplement the article with what was forgotten from the very beginning!
And yes - I don’t have the time and energy to build a “hit parade”, so it will be a hodgepodge. And with all the exceptions, it turned out to be quite short.
Caustic alkalis
Specifically, alkali metal hydroxides: lithium, sodium, potassium, rubidium, cesium, francium, thallium (I) hydroxide and barium hydroxide. But:
- Lithium, cesium, rubidium and barium are discarded - expensive and rare
- If you, %username%, come across francium hydroxide, then the last thing you will worry about is causticity - it is terribly radioactive
- It’s the same with thallium - it’s terribly poisonous.
Therefore, sodium and potassium remain.
But let's be honest - the properties of all caustic alkalis are very similar. Sodium hydroxide is known to everyone as “caustic soda” (not to be confused with baking soda, soda ash and other soda, as well as potash). Potassium hydroxide as a food additive E525 too. Both are similar in properties: they are highly hygroscopic, that is, they attract water and “dissolve” in air. They dissolve well in water and release a large amount of heat.
“Spreading” in air is essentially the formation of very concentrated solutions of alkalis. Therefore, if you put a piece of caustic alkali on paper, leather, some metals (the same aluminum) - then after a while you will find that the material has eaten well! What was shown in “Fight Club” is very similar to the truth: indeed, sweaty hands - and the alkali - will hurt! Personally, I found it more painful than hydrochloric acid (more on that below).
However, if your hands are very dry, most likely you won’t feel anything in the dry alkali.
Caustic alkalis are excellent at breaking down fats into glycerin and salts of fatty acids - this is how soap is made (hello, “Fight Club!”) A little longer, but just as effectively, proteins are broken down - that is, in principle, alkalis dissolve flesh, especially strong solutions - and when heated . The disadvantage in comparison with the same perchloric acid (more on that below) is that all alkalis draw carbon dioxide from the atmosphere, and therefore the strength will gradually decrease. In addition, alkalis also react with the components of glass - the glass becomes cloudy, although in order to dissolve it completely, here, of course, you have to try.
Tetraalkylammonium hydroxides are sometimes classified as caustic alkalis, for example
tetramethylammonium hydroxide
In fact, these substances combine the properties of cationic surfactants (well, it’s like ordinary soap - only cationic: here the active diphilic particle is with a “+” charge, and in soap - with a “-” charge) and relatively high basicity. If it gets on your hands, you can lather it in water and wash it like soap; if you warm your hair, skin or nails in an aqueous solution, they will dissolve. The “causticity” against the background of sodium and potassium hydroxides is so-so.
Sulfuric acid
H2SO4
The most popular, probably, in all stories. Not the most caustic, but quite unpleasant: concentrated sulfuric acid (which is 98%) is an oily liquid that loves water very much, and therefore takes it away from everyone. By taking water away from cellulose and sugar, it chars them. In the same way, she will happily take the water away from you, %username%, especially if you pour it on the delicate skin of your face or into your eyes (well, in fact, everything will get into your eyes with adventure). Particularly kind people mix sulfuric acid with oil to make it harder to wash off and better absorbed into the skin.
By the way, when taking in water, sulfuric acid heats up greatly, which makes the picture even more juicy. Therefore, washing it off with water is a very bad idea. It’s better to use oil (rinse off, not rub in, and then rinse with water). Well, or a large flow of water to immediately cool it.
“First water, and then acid - otherwise big trouble will happen!” — this is specifically about sulfuric acid, although for some reason everyone thinks that it’s about any acid.
Being an oxidizing agent, sulfuric acid oxidizes the surface of metals to oxides. And since the interaction of oxides with acids takes place with the participation of water as a catalyst - and sulfuric acid does not release water - an effect called passivation occurs: a dense, insoluble and impenetrable film of metal oxide protects it from further dissolution.
According to this mechanism, concentrated sulfuric acid is sent to distant distances by iron and aluminum. It is noteworthy that if the acid is diluted, water appears, and it is impossible to send - the metals dissolve.
By the way, sulfur oxide SO3 dissolves in sulfuric acid and produces oleum - which is sometimes mistakenly written as H2S2O7, but this is not entirely correct. Oleum has an even greater attraction to water.
My own feelings when sulfuric acid gets on my hand: it’s a little warm, then it burns a little - I washed it off under the tap, no big deal. Don't believe the movies, but I don't recommend putting it on your face.
Organics often use chromium or “chromic mixture” - this is potassium dichromate dissolved in sulfuric acid. Essentially this is a solution of chromic acid, it is good for washing dishes from organic residues. If it gets on your hand, it also burns, but essentially it’s sulfuric acid plus toxic hexavalent chromium. You won't find holes in your hand, except maybe on your clothes.
The author of these lines knows an idiot who used potassium permanganate instead of potassium dichromate. Upon contact with organic matter, it stung a little. Those present shit themselves and escaped with a slight fright.
By the way, since we remembered chromium, let’s digress a little from the topic of acids and
Chromyl chloride
CrO2Cl2
Essentially, it is a violent compound of hexavalent chromium and hydrochloric acid. The dark red liquid, which draws water, hydrolyzes - and eventually smokes this very hydrochloric acid. Causticity is the result of this fraternal unity: chromium oxidizes, hydrochloric acid dissolves: it ignites some organic solvents (alcohol, turpentine), but dissolves in some (carbon tetrachloride, dichloromethane, carbon disulfide). It eats up metals, but not as well as acids - again it’s a matter of passivation. for example, steel, when exposed, acquires a beautiful dark blue surface.
The skin - understandably - ulcerates, and chrompica is stronger in this, since it penetrates better into the skin as into non-polar organic tissue. But it’s not even that, it’s about hexavalent chromium, which is actually a carcinogen, and therefore if it penetrates deeper, there are more problems. And of course, inhaling is much more dangerous.
Hydrochloric acid
HCl
does not exceed 38% in water. One of the most popular acids for dissolution - in this it is cooler than others, because technologically it can be very pure, and in addition to acting as an acid, it also forms complex chlorides that increase solubility. By the way, it is for this reason that insoluble silver chloride is very soluble in concentrated hydrochloric acid.
This one, when it comes into contact with the skin, burns a little more, subjectively, it also itches, and also stinks: if you work a lot with concentrated hydrochloric acid in a laboratory with a poor hood, your dentist will thank you: you’ll make it rich on fillings. By the way, chewing gum helps. But not much. Better - a hood.
Since it is not oily and does not heat up much with water, it is caustic only to metals, and not to all. By the way, steel in concentrated hydrochloric acid is passivated and says “nope!” This is what they use during transportation.
Nitric acid
HNO3
Also very popular, for some reason they are also afraid of it - but in vain. Concentrated - this is the one up to 70% - it is the most popular, higher - it is “smoking”, most often no one needs it. There is also anhydrous one - and it also explodes.
Being an oxidizing agent, it passivates many metals that become covered with an insoluble film and say “goodbye” - these are chromium, iron, aluminum, cobalt, nickel and others.
It instantly reacts with the skin according to the principle of the xanthoprotein reaction - there will be a yellow spot, which means that you, %username%, are still made of protein! After some time, the yellow skin will peel off, as if burned. At the same time, it stings less than salt, although it stinks no worse - and this time it is more toxic: flying nitrogen oxides are not very good for the body.
In chemistry, they use the so-called “nitrating mixture” - the most popular one consists of sulfuric and nitric acids. It is used in syntheses, in particular in the production of a cheerful substance - pyroxylin. In terms of causticity - the same chromium plus beautiful yellow skin.
There is also “regia vodka” - this is part nitric acid to three parts hydrochloric acid. Used to dissolve certain metals, mainly precious ones. The drip method of checking the sample of gold products is based on different ratios and the addition of water - by the way, it is very difficult for specialists using this method to fool with a fake. In terms of causticity for the skin - the same “nitrating mixture” plus it smells great, the smell cannot be confused with anything else, it is also quite toxic.
There is also “reverse aqua regia” - when the ratio is reversed, but this is a rare specificity.
By the way, about that very “smoking” one, which is red, angry and an oxidizing agent - I quote the story of a good friend who sent me just now.
I drove this very 98% nitrogen. Whether it was simply distilled for purification, or from melange, I don’t remember. I caught up with about two liters and removed the receiver. I ask the laboratory assistant to give me a clean 2-liter flask and pour it into it. She gave it to me, dry, clean, but with alcohol - and with the cap closed. That is, the pairs were and accumulated. I pour it into the funnel and pour it. I take her there and she comes back. I sprayed it well on my hands, on my face and below my neck. It felt like an eagle grabbed your face. Plus the arms, neck, under the nose, etc. little things. In my hands, I remind you, are two liters of the same goodness. Eyes closed, naturally. I understand that you can’t throw the flask, it will immediately become much worse. I carefully place the flask on the rubber stand, move to the sink, turn the gander into my face and turn on full pressure. I managed it in about five seconds. I didn’t reach the subcutaneous tissue. Otherwise everything would have been much worse. I saw with another guy that it happens after 10-15 seconds. Difficult-to-heal purple scars on half of the arm. Then I realized why she was so angry. Not only is it a fairly strong acid and oxidizing agent, it is also a wonderful solvent. It is indefinitely miscible with water, but also indefinitely miscible with, for example, dichloroethane. This is some kind of biphilic rubbish.
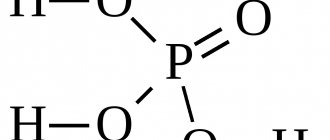
Phosphoric acid
H3PO4
In fact, I gave the formula for orthophosphoric acid, the most common one. And there is also metaphosphoric, polyphosphoric, ultraphosphoric - in short, that’s enough, but it doesn’t matter.
Concentrated orthophosphoric acid (85%) is such a syrup. The acid itself is average, it is often used in the food industry, by the way - when you get fillings, the surface of the tooth is first etched with phosphoric acid.
Its corrosion properties are so-so, but there is an unpleasant nuance: this syrup is well absorbed. Therefore, if it drips on things, it will be absorbed, and then it will slowly corrode. And if there is a stain or a hole from nitric and hydrochloric acid, then from phosphorus the thing will fall apart, this is especially colorful on shoes, when the hole seems to crumble until it turns out right through.
Well, in general it’s difficult to call it caustic.
Hydrofluoric acid
HF
Concentrated hydrofluoric acid is about 38%, although there are the odd exception.
A weak acid that takes the fierce love of fluoride ions to form persistent complexes with everyone with whom it can. Therefore, it surprisingly dissolves what other, stronger friends cannot, and therefore is very often used in various mixtures for dissolution. When you get it on your hand, the sensations will be greater from other components of such mixtures, but there is a nuance.
Hydrofluoric acid dissolves SiO2. That is sand. That is glass. That is, quartz. And so on. No, if you splash this acid on a window, it won’t dissolve, but a cloudy stain will remain. To dissolve, you need to hold it for a long time, or even better, heat it. When dissolved, SiF4 is released, which is so beneficial for health that it is better to do it under a hood.
A small but pleasant nuance: you, %username%, contain silicon in your nails. So, if hydrofluoric acid gets under your nails, you won’t notice anything. But you won’t be able to sleep at night - it will hurt SO much that sometimes you want to tear your finger off. Believe me, friend, I know.
And in general, hydrofluoric acid is toxic, carcinogenic, absorbed through the skin and a lot of other things - but today we’re talking about causticity, right?
Do you remember how we agreed at the very beginning that there would be no fluoride? He won't be. But they will...
Fluorides of inert gases
In fact, fluorine is a tough guy, you can’t really show off with it, and therefore some inert gases form fluorides with it. The following stable fluorides are known: KrF2, XeF2, XeF4, XeF6. All these are crystals, which in air at different speeds and readily decompose with moisture to hydrofluoric acid. The causticity is appropriate.
Hydroiodic acid
HI
The strongest (by degree of dissociation in water) binary acid. A strong reducing agent, which is used by organic chemists. In air it oxidizes and turns brown, which causes stains upon contact. The sensation upon contact is like salt water. All.
Perchloric acid
HClO4
One of the strongest (in terms of the degree of dissociation in water) acids in general (super acids compete with it - more about them below) - the Hammett acidity function (a numerical expression of the ability of a medium to be a proton donor in relation to an arbitrary base, the lower the number, the stronger the acid) is -13. Anhydrous is a strong oxidizing agent, likes to explode, and is generally unstable. Concentrated (70%-72%) is an oxidizing agent no worse, often used in the decomposition of biological objects. Decomposition is interesting and exciting because it can explode in the process: you need to make sure that there are no coal particles, that it does not boil too violently, etc. Perchloric acid is also quite dirty - it cannot be purified by subdistillation, the infection explodes! Therefore, it is not used often.
When it comes into contact with the skin, it burns and feels like salt. It stinks. When you see in films that someone threw a corpse into a container with perchloric acid and it dissolved, then yes, this is possible - but it will take a long time or warm it up. If you heat it up, it may explode (see above). So be critical of cinema (I think I saw this in 10 Cloverfield Lane).
By the way, the causticity of chlorine oxide (VII) Cl2O7 and chlorine oxide (VI) Cl2O6 is the result of the fact that these oxides form perchloric acid with water.
Now let’s imagine that we decided to combine strong acidity and the causticity of fluorine in one compound: let’s take a molecule of perchloric or sulfuric acid and replace all the hydroxyl groups on it with fluorine! The rubbish will turn out to be rare: it will interact with water and similar compounds - and at the site of the reaction a strong acid and hydrofluoric acid will immediately be obtained. A?
Fluorides of sulfur, bromine and iodine
Remember we agreed to consider only liquids? For this reason, our article did not include chlorine trifluoride ClF3
, which boils at +12 °C, although all the horror stories are that it is terribly toxic, ignites glass, a gas mask and, when spilled 900 kilograms, eats 30 cm of concrete and a meter of gravel - everything This is true. But we agreed - liquids.
However, there is a yellow liquid - iodine pentafluoride IF5
, colorless liquid -
bromine trifluoride BrF3
, light yellow -
bromine pentafluoride BrF5
, which are no worse. BrF5, for example, also dissolves glass, metals and concrete.
Similarly, among all sulfur fluorides, only disulfur decafluoride (sometimes called sulfur pentafluoride) is liquid - a colorless liquid with the formula S2F10
. But this compound is quite stable at ordinary temperatures, does not decompose with water - and therefore is not particularly caustic. True, it is 4 times more toxic than phosgene with a similar mechanism of action.
By the way, iodine pentafluoride is said to have been the “special gas” used to fill the atmosphere in the escape shuttle in the final scenes of the 1979 film Alien. Well, I don’t remember, honestly. Reminds me! Damn, it’s so cool there that I couldn’t resist and dedicated a separate article to it.
I even found it, took a closer look and realized that Ripley lived there in such harsh conditions that the alien beast was just cute
Super acids
The term "super acid" was coined by James Conant in 1927 to classify acids that are stronger than ordinary mineral acids.
In some sources, perchloric acid is classified as a super acid, although this is not so - it is an ordinary mineral one. A number of superacids are mineral acids to which a halogen has been attached: the halogen pulls electrons onto itself, all the atoms get very angry, and everything goes to hydrogen as usual: it falls off in the form of H+ - boom: so the acid has become stronger.
Examples - fluorosulfuric and chlorosulfuric acids
Fluorosulfuric acid has a Hammett function of -15.1; by the way, thanks to fluorine, this acid gradually dissolves the test tube in which it is stored.
Then someone smart thought: let's take a Lewis acid (a substance that can accept a pair of electrons from another substance) and mix it with a Brønsted acid (a substance that can donate a proton)! Antimony pentafluoride was mixed with hydrofluoric acid to obtain hexafluorantimony acid HSbF6
. In this system, hydrofluoric acid releases a proton (H+), and the conjugate base (F−) is isolated by a coordination bond with antimony pentafluoride. This produces a large octahedral anion (SbF6−), which is a very weak nucleophile and a very weak base. Having become “free”, the proton determines the hyperacidity of the system - Hammett function -28!
And then others came and said why they took Bernstead’s weak acid and came up with this.
Trifluoromethanesulfonic acid
- in itself is already a super acid (Hammett function -14.1).
So, they added antimony pentafluoride to it again - they got a decrease to -16.8! The same trick with fluorosulfuric acid gave a reduction to -23. And then a group of scientists from the chemistry department of the American University of California, led by Professor Christopher Reed, hung out with colleagues from the Institute of Catalysis of the Siberian Branch of the Russian Academy of Sciences (Novosibirsk) and came up with carborane acid H(CHB11Cl11). Well, they called it “carborane” for ordinary people, but if you want to feel like a scientist, say “2,3,4,5,6,7,8,9,10,11,12-undecachlor-1-carba-closo-dodecaborane (12)” three times and quickly.
This is what this beauty looks like
This is a dry powder that is soluble in water.
This is the Strongest Acid at the moment. Carborane acid is approximately a million times stronger than concentrated sulfuric acid. It is not possible to measure the strength of an acid on a conventional scale, since the acid protonates all known weak bases and all solvents in which it dissolves, including water, benzene, fullerene-60, and sulfur dioxide. Subsequently, Christopher Reed told Nature news service: “The idea for the synthesis of carborane acid was born out of fantasies about “molecules that had never been created before.” Together with his colleagues, he wants to use carborane acid to oxidize atoms of the noble gas xenon - simply because no one has done it before. Original, what can I say.
Well, since super acids are ordinary acids, they act normally, only a little stronger. It is clear that the skin will burn, but this does not mean that it will dissolve. Fluorosulfonic acid is a separate case, but it’s all thanks to fluorine, just like in fluoride.
Trihaloacetic acids
Specifically, trifluoroacetic and trichloroacetic acid
Cute and pleasant due to the combination of the properties of an organic polar solvent and a fairly strong acid.
They stink—like vinegar. The cutest thing is trifluoroacetic acid: a 20% solution destroys metals, cork, rubber, bakelite, polyethylene. The skin burns and forms dry ulcers that reach the muscle layer.
Trichloroacetic acid is the younger brother in this regard, but that’s okay too. By the way, applause to the weaker sex: in pursuit of beauty, some go for the so-called TCA peeling procedure (TCA is TetraChloroAcetate) - when this same trichloroacetic acid is used to dissolve the top, rough layer of skin.
True, if a cosmetologist chats on the phone, a failure is possible
Acetic acid
CH3COOH
Most likely, you have this acid in your kitchen - and yes, it is used as a food additive E260. But it can also be stronger - a 70-80% aqueous solution of acetic acid is called acetic essence, and if the concentration is close to 100% - glacial acetic acid (because it can freeze and form something similar to ice.
Acetic acid is not as caustic towards metals as mineral acids, but since it is not so polar, and to some extent even diphilic (a combination of hydrophobic and hydrophilic parts in one molecule - as in surfactants), it is great absorbed by the skin. Solutions with acetic acid concentrations greater than 30% are considered dangerous. The peculiarity of burns is that the development of coagulation necrosis of adjacent tissues of varying length and depth is also initiated - if not washed off, there will be long-healing ulcers and scars.
Well, it stinks, of course, quite noticeably.
Formic acid
HCOOH
We have already discussed that formic acid, which is formed in the body after taking methanol, is one of the main reasons for its toxicity. So, formic acid from the outside is not at all so dangerous, since it is quickly metabolized and eliminated by the body. Toxicity is quite low - for rats LD50 is about 1.8 g / kg, and therefore formic acid is also often used, including as a food additive - and there is no need to be afraid of this.
The “causticity” of formic acid depends on the concentration. According to the classification of the European Union, a concentration of up to 10% has an irritating effect, and more than 10% has a corrosive effect. And again we are not talking about metals and glass - but about the body. Upon contact with skin, 100% liquid formic acid causes severe chemical burns. Contact of even a small amount of it on the skin causes severe pain; the affected area first turns white, as if covered with frost, then becomes wax-like, with a red border appearing around it. The acid easily penetrates the fatty layer of the skin, so washing the affected area with a soda solution must be done immediately. So the ants really do know something.
Bromine
Br2
A heavy, caustic liquid of red-brown color with a strong unpleasant odor, vaguely reminiscent of the smell of both iodine and chlorine. By the way, the name “bromine” comes from the Greek βρῶμος - “stinker”, “smelly”.
Bromine is a typical halogen; in terms of chemical activity, bromine occupies an intermediate position between chlorine and iodine. That is, not as fast as fluorine - but livelier than boring iodine. And yes, it doesn’t reach chlorine either.
Slightly soluble in water, well soluble in some organic solvents. Bromine water, a reagent for unsaturated hydrocarbons, stinks, but is quite peaceful and does not dissolve anything much.
Pure bromine is powerful, smelly and hairy, as well as toxic. When it comes into contact with the skin, it causes burns: the trouble is that bromine molecules are non-polar, and therefore penetrate well into hydrophobic human skin and flesh - and therefore burns are really painful, take a long time to heal, and almost always leave a scar. Aluminum flares up on contact with bromine, other metals are more temperate, but in powder form - some react, for example, iron.
Concrete and glass are quite resistant to bromine. Organic compounds with bromine - what? - Right! - are brominated in the presence of an unsaturated bond. For this reason, the stability of polymers depends on their type, for example polyethylene and polypropylene - they didn’t care about bromine at room conditions.
Hydrogen peroxide
H2O2
An unstable compound that constantly breaks down gradually into oxygen and water. The higher the concentration, the more unstable it is, which gradually turns into an explosive hazard. To stabilize technical hydrogen peroxide, sodium pyrophosphate or sodium stannate is added to it; When stored in aluminum containers, a corrosion inhibitor is used - ammonium nitrate.
Hydrogen peroxide in the laboratory is usually a 38% solution. Upon contact with skin, it causes a chemical burn with a characteristic white color. The burn is painful, especially on thin skin; the whitened, keratinized skin then often cracks and itches.
In medicine, 3% hydrogen peroxide is used to clean deep wounds with a complex profile, purulent streaks, phlegmons and other purulent wounds, the sanitation of which is difficult - the substance not only has an antiseptic effect, but also creates a large amount of foam when interacting with the enzyme catalase. This, in turn, makes it possible to soften and separate necrotic areas, blood clots, and pus from the tissues, which will be easily washed away by subsequent injection of an antiseptic solution into the wound cavity. By the way, hydrogen peroxide is undesirable in other cases of wounds: while having good cleansing properties, this substance does not actually speed up the healing process, since it damages the cells adjacent to the wound, as well as young, newly formed tissues - and this is also fraught with the formation of scars.
Except for burns on the skin, it does not corrode or dissolve anything. Metals, glass and plastics are resistant to hydrogen peroxide.
Hydrogen peroxide also gave the world many unique natural blondes with black hair roots!
Close to hydrogen peroxide are the so-called peracids - acids that contain peroxide groups. Example: peracetic acid CH3COOOH
- a substance that resembles hydrogen peroxide in its properties, and therefore is used in exactly the same areas.
There is “pervomur” or “S-4” (no, this is not the C-4 you thought about) - this is performic acid HCOOOH
, which is even weaker than peracetic, and therefore surgeons wash their hands with it before surgery.
And finally - trifluoroperacetic acid CF3COOOH
- a fierce, crazy oxidizing agent, which organic chemists look at with admiration for the possibility of oxidizing aniline to nitrobenzene, obtaining hypervalent iodine in organic compounds, the Bayer-Villiger reaction and other things that are poorly understood by normal people. In terms of causticity - trifluoroacetic acid mixed with hydrogen peroxide, which, in fact, is what it is, and therefore poses a particular danger to the hands, yes. Due to its high oxidizing power, trifluoroperacetic acid is not sold, but is usually obtained by admiring organic chemists directly, where necessary, by reacting trifluoroacetic anhydride with hydrogen peroxide.
Well, something like this, if we talk about liquid and causticity. Will there be more additions?
Formula
Phosphoric acid is an inorganic compound that is described by the formula H3PO4. Its molar mass is 98 g/mol. A microparticle of a substance is built in space in such a way that it connects hydrogen and oxygen atoms with each other. The formula shows that the chemical substance has the following composition:
| Number of atoms | Mass percentage | |
| Hydrogen | 3 | 3,1 |
| Phosphorus | 1 | 65,3 |
| Oxygen | 4 | 31,6 |
Physical properties
Phosphoric (orthophosphoric) acid with a molar mass of 97.99 g/mol and the empirical formula H3PO4 is an inorganic tribasic acid of medium strength. The structural formula of the molecule in the gaseous aggregate state is described in the form of a tetrahedron, containing a phosphorus atom in the center, and an oxygen atom and three hydroxyl groups at the vertices.
The composition is as follows:
| Name | Number of atoms | Mass fraction, % |
| Hydrogen (H) | 3 | 3,10 |
| Phosphorus (P) | 1 | 65,30 |
| Oxygen (O) | 4 | 31,60 |
Under normal conditions, the colorless crystals are hygroscopic, melt in air already at 42.35 °C, and easily dissolve in water, ethyl alcohol and other solvents. Three types of aqueous solutions have practical application:
| Concentration, % | Melting point, °C | Density, gram/ml |
| 75 | -20 | 1,579 |
| 80 | 0 | 1,633 |
| 85 | +20 | 1,689 |
A colorless and odorless syrupy liquid with an 85% concentration of H3PO4 is usually called orthophosphoric acid, and by boiling in a vacuum at 80 °C the anhydrous component is released from it. In the solid phase and in highly concentrated solutions, phosphoric acid molecules form intermolecular hydrogen bonds.
When diluting, hydrogen bonds between phosphate anions PO43- and water molecules H2O come to the fore.
Preparation of phosphoric acid
A chemical compound has several production methods. The well-known industrial method for producing phosphoric acid is thermal, which produces a pure, high-quality product. The following process occurs:
- oxidation during combustion with excess air of phosphorus to phosphorus anhydride having the formula P4O10;
- hydration, absorption of the resulting substance;
- condensation of phosphoric acid;
- capturing mist from the gas fraction.
There are two more methods for producing orthophosphorus compounds:
- An economical extraction method. Its basis is the decomposition of natural phosphate minerals with hydrochloric acid.
- Under laboratory conditions, the substance is obtained by reacting white phosphorus, which is toxic, with dilute nitric acid. The process requires strict adherence to safety precautions.
How is it produced?
Phosphoric acid can be synthesized either by a wet method or by a thermal method.
The latter uses air, water and elemental phosphorus as raw materials. It includes three important stages: combustion, humidification and fogging. Here's how it happens:
First, liquid elemental phosphorus is burned in a combustion chamber at high temperatures ranging from 1650 to 2760 °C. This oxidation reaction occurs in ambient air and produces phosphorus pentoxide.
4P + 5O 2 -> 2 P 2 O 5
The product is then hydrated with water to form strong phosphoric acid (in liquid form).
P 2 O 5 + 6 H 2 O -> 2 P 2 O 5
At the last stage, high-pressure mist eliminators are used to remove phosphoric acid mist from the flue gas stream.
The concentration of phosphoric acid synthesized in this process is typically between 75 and 85 percent. These concentration levels are necessary to produce high quality chemical products. Several efficient plants recover extremely concentrated phosphoric acid (up to 99.9%) using the same thermal process.
However, almost 80% of phosphoric acid is produced by the wet process. In this method, sulfuric acid is treated with a natural phosphate-containing mineral such as hydroxyapatite.
Ca 5 (PO 4) 3 OH + 5 H 2 SO 4 -> 3 H 3 PO 4 + 5 CaSO 4 + H 2 O
The mineral is dried, crushed, and then fed into the reactor along with sulfuric acid. The reaction fuses the sulfate with calcium (from the mineral) to form calcium sulfate (gypsum).
Treated water is then added and the gypsum, along with other insoluble impurities, is removed through a filter. Phosphoric acid obtained by this wet method contains 25-30% phosphorus pentoxide.
Typically, this acid is further concentrated to meet the requirements for fertilizer production. In most cases, phosphoric acid is concentrated to 40-55% phosphorus pentoxide using two/three vacuum evaporators.
Chemical properties
The inorganic compound is considered tribasic and of medium strength. The following chemical properties of orthophosphoric acid are characteristic:
- reacts to indicators by changing color to red;
- when heated, it is converted to pyrophosphoric acid;
- in aqueous solutions undergoes three-stage dissociation;
- when reacting with strong acids, it forms phosphoryls - complex salts;
- forms a yellow precipitate when interacting with silver nitrate;
- thermally decomposes to diphosphoric acid;
- upon contact with bases, amorphous hydroxides, it forms water and salt.
Cleaning rust by applying orthophosphoric acid to the surface
If a large part of the car has suffered from rust and it is not possible to immerse it completely in the solution. Or the amount of reagent is very limited, another method can be used.
Here, too, it is necessary to clean and degrease the surface. If the degree of rust is high, you may need to use a grinder. The attachment can be a metal brush. Next, orthophosphoric acid is applied in a convenient way. After a few hours the surface can be neutralized and cleaned. You should be careful with decorative coatings on metal; it is not friendly to all of them.
Advice!
Phosphoric acid interacts not only with oxidized iron, but also with the rest of the metal. This can cause thinning and holes in the surface. This process can be stopped using the inhibitor "Katapin". Per liter of water, 1 gram of this neutralizing substance is required.
Application
Phosphoric acid is used in many areas, from industry to dental treatment. The product is used by craftsmen as a flux when soldering, to clean the metal surface from rust. Liquid is used:
- for scientific research in molecular biology;
- as a catalyst for organic synthesis processes;
- for creating anti-corrosion coatings on metals;
- in the production of fire-resistant impregnations for wood.
The substance is used:
- in the oil industry;
- in the manufacture of matches;
- for film production;
- for the purpose of protection against corrosion;
- to clarify sucrose;
- in the manufacture of medicines;
- in refrigeration units as a binder in freon;
- during mechanical processing for polishing and cleaning metals;
- in the textile industry in the production of fabrics with fire retardant impregnation;
- as a component in the production of chemical reagents;
- in veterinary medicine for the treatment of urolithiasis in minks;
- as a component for metal primer.
In the food industry
The use of phosphoric acid in food production has become widespread. It is registered in the register of food additives under code E338. When consumed in acceptable quantities, the substance is considered safe. The following properties of the drug are useful:
- preventing rancidity;
- acidity regulation;
- shelf life extension;
- preservation of taste characteristics;
- enhancing the effect of antioxidants.
Phosphoric acid as an acidifier, leavening agent, and antioxidant is used in the bakery, meat, and dairy industries. Used in the production of confectionery and sugar. The substance gives products a sour, bitter taste. Additive E338 is included in:
- processed cheeses;
- muffins;
- carbonated drinks - Pepsi-Cola, Sprite;
- sausages;
- buns;
- milk;
- baby food;
- marmalade;
- cakes
Research has shown that overconsumption of products containing phosphorus compounds, especially carbonated drinks, can lead to health problems. It is possible:
- leaching of calcium from the body, which can trigger the formation of osteoporosis;
- violation of the acid-base balance - the additive can increase its acidity;
- the appearance of gastrointestinal diseases;
- exacerbation of gastritis;
- destruction of tooth enamel;
- development of caries;
- the appearance of vomiting.
In the non-food industry
The use of phosphoric acid can be observed in many areas of production. This is often due to the chemical properties of the product. The drug is used for the manufacture of:
- combined, phosphorus mineral fertilizers;
- activated carbon;
- phosphorus salts of sodium, ammonium, manganese;
- fire retardant paints;
- glass, ceramics;
- synthetic detergents;
- fire-resistant binding components;
- non-flammable phosphate foam;
- hydraulic fluids for the aviation industry.
- Shortbread cookie dough: recipes with photos
- Eyebrow microblading - what is it?
- Home dough mixer
In medicine
Dentists use orthophosphorus composition to treat the inner surface of the crown. This helps improve its adhesion to the tooth during prosthetics. The substance is used by pharmacists to prepare medicines and dental cement. In medicine, the use of orthophosphorus compounds is associated with the ability to etch tooth enamel. This is necessary when using adhesive materials of the second or third generation for filling. Important points - after etching the surface it is necessary to:
- Rinse;
- dry.
Harm to the body
Officially, phosphoric acid is recognized as completely safe for human health and is approved for use in different countries around the world, but scientific practical data indicate the presence of harmful consequences for the body of living beings. The product is non-toxic and does not lead to negative consequences when consumed in small quantities. It can cause the development of caries, which arises from a violation of the integrity of the top layer of enamel when it comes into contact with sugar crystals. The resulting environment is favorable for the life of bacteria in the oral cavity. Phosphoric acid is in demand in the production of carbonated drinks, baked goods, sausages and processed cheeses, the use of which leads to a violation of the acidity level. The body's protective functions begin to restore the alkaline balance using calcium taken from bones and teeth. A lack of calcium compounds leads to pain in the joints and destruction of the integrity of the teeth. A solution of orthophosphoric acid with a concentration of more than 50%, if the technology and process control are not followed, causes:
- burns of mucous membranes and skin;
- the occurrence of bloody discharge from the nose;
- dental problems;
- abnormalities in the blood count;
- vomiting;
- diarrhea caused by a malfunction of the digestive tract.
With prolonged action of a food additive on the body, a person loses the sense of appetite, which leads to sudden weight loss and health problems. If you have diseases of the intestinal tract, the use of products with the food additive E338 should be completely excluded from the diet.
Anti-rust application
A rust converter based on phosphoric acid creates a protective layer on the surface that protects against corrosion during further use. The peculiarity of using the compound is that it is safe for metal when applied. There are several ways to remove rust with phosphoric acid, depending on the size of the damage:
- etching with immersion in a bath or other container;
- repeated application of the composition to the metal with a spray gun or roller;
- covering the surface with pre-treated mechanical cleaning.
The orthophosphorus compound converts rust into iron phosphates. The composition can be used for washing and cleaning:
- rolled metal products;
- wells;
- pipeline surfaces;
- steam generators;
- water supply, heating systems;
- coils;
- boilers;
- water heaters;
- heat exchangers;
- boilers;
- machine parts and mechanisms.
Use of acid
The use of phosphoric acid is quite wide. It is worth considering the most popular methods of using it.
In medicine
It is used in dentistry during tooth filling to etch the enamel immediately before the start of the process. This procedure has its negative aspects, since it is impossible to control the depth and stage of enamel splitting, as well as their complete removal before filling. The substance remaining after such a procedure can reduce the strength of the protection and lead to the formation of acidic residues on the tooth enamel. This acid is added in small doses to tooth whiteners.
Removing rust from metal surfaces using the submersible method and surface application. Rust converter
The advantage of removing rust with phosphoric acid is that it removes corrosion from the metal and creates a thin film on them, protecting them from various external influences. After covering a metal surface with this substance, an active process of corrosion and absorption of iron oxide begins. Then a gray film of an oily consistency forms on the metal plane.
There are various methods for removing oxides, among which are the following:
- with complete immersion of the element in an acid solution;
- surface treatment using a spray, brush or roller;
- coating with a solution of a mechanically pre-treated top layer of metal.
The corrosion converter is an acid solution with various additives. There are these types of solutions, depending on the additives used in their composition:
- primers;
- modifiers-stabilizers;
- rust converters.
Type 1 includes primer EVA-0112, consisting of the main component and 85% solution of the substance. It acts as a base for painting.
The Tsinkar converter contains acid and salts of manganese and zinc. When used, rust is transformed into a dense protective layer. The alloying process takes place.
Phosphoric acid for metal
To clean or solder metal elements, you must perform the following operations. Before completely immersing a metal element in an orthophosphorus composition, it is first cleaned of various types of deposits on the surface, in particular fats. To do this, wash the part using a cleaning agent. After this, it is necessary to dissolve 150 ml of the substance in 1 liter of water and immerse the metal element in this solution for 1 hour, stirring the liquid from time to time for greater efficiency.
Then you need to wash off the mixture with a solution that consists of 50% water, 2% ammonia and 48% ethanol. After this, the element must be rinsed under running water and dried well.
Before applying the spray to the surface with a roller or brush, you should first clean the surface from rust. After application, you should wait a little, and then wash off the mixture with a neutralizing solution and dry the part.
Such procedures can be carried out with different metals, including aluminum.
Application in agriculture
In agriculture, phosphoric acid extracted from the ore is used as a fertilizer. When it gets into the soil and then into plants, it helps them withstand drought and frost. At the same time, the soil becomes more fertile and favorable for growing vegetables and herbs.
Use of acid in everyday life and food industry
The use of acid in everyday life implies its use to remove corrosion from various surfaces (with the exception of acrylic). It is suitable for processing enamel and earthenware surfaces. Before applying the orthophosphoric acid solution, the metal surface must be treated with a detergent. To prepare the solution, mix 1 liter of water and 200 g of the active substance, and then apply the mixture to the surface to be treated for 1-12 hours. After time, the mixture must be extinguished with a soda solution and washed off.
In food production it is used as an acidity regulator.
Interaction of phosphoric acid
The properties of an inorganic substance determine its interaction with other substances and compounds. In this case, chemical reactions occur. The orthophosphorus composition interacts with:
- salts of weak acids;
- hydroxides, entering into a neutralization reaction;
- metals to the left of hydrogen in the activity series with the formation of salt and the release of hydrogen;
- basic oxides, participating in the exchange reaction;
- ammonium hydroxide, creating ammonium hydrogen phosphate;
- ammonia to produce acid salts.
How to clean rust using phosphoric acid at home?
Cleaning must be done extremely carefully, otherwise the metal may become thinner or even holes may form on the car. Therefore, it is necessary to limit the degree of exposure of orthophosphoric acid to the metal. When preparing the surface for processing, grinding discs with too strong abrasive properties should not be used. This can damage the surface, which will no longer be able to withstand subsequent cleaning.
Before application, it is better to protect the rest of the surface from exposure to such a strong substance as orthoosphoric acid. Accuracy and precision of the impact will help to avoid additional expenses for the restoration of a surface that is not rusted, but has been damaged by acid.
If everything is done correctly, the surface will be completely free of rust, which would sooner or later destroy the metal. Phosphoric acid can be harmful to the human body, so all work with it is carried out with strong gloves and a mask. If phosphoric acid gets on your clothing, it is better to remove it immediately to stop the poison from getting on your skin.
There are three main methods of using phosphoric acid to remove rust from metal parts. The choice of method depends on the size, degree of damage and other properties of the item being cleaned. Cleansing can be achieved by completely immersing the object in an acidic environment. The surface can be sprayed with phosphoric acid from a spray bottle or applied with a roller. Rust can be removed mechanically by then treating the part with acid.
Safety precautions when working with acid
Orthophosphorus compound belongs to the class of hazardous substances and requires caution. Work with the composition must be carried out in a special room equipped with supply and exhaust ventilation, away from sources of fire. The lack of personal protective equipment is unacceptable:
- respirator;
- gloves;
- special clothing;
- non-slip shoes;
- points.
Contact of orthophosphorus composition on the skin or eyes is dangerous, and inhalation of hot vapors is harmful. This may cause burns, dizziness, vomiting, and coughing. In case of emergency you need to:
- remove clothing that has come into contact with the substance;
- rinse the affected area with running water;
- call a doctor;
- apply a loose bandage;
- Neutralize spilled liquid with alkali.
Precautionary measures
When working with any acids, the most important thing is your own safety. Phosphoric acid is no exception. Before using it, you need to make sure you have a prepared respirator and rubber gloves. After all, phosphoric acid is a rather dangerous chemical that causes skin burns. The fumes of this acid are no less dangerous: their action can lead to severe poisoning or burns of the respiratory tract. It is also worth remembering that phosphoric acid is highly flammable and can lead to a fire. It is for these reasons that most activities with this substance should be carried out outdoors or in well-ventilated rooms. The main thing is not to let the acid get on the skin, but if this does happen, you should immediately wash the affected area under running water. If a significant part of the skin has been exposed to a chemical burn, you should immediately consult a doctor.
How to defeat scale? Advice from experienced housewives
To remove rust and scale at home, use a weak solution of phosphoric acid. It turns rust into a black coating, which can then be easily cleaned from a metal product. Orthophosphoric acid is also indispensable for removing scale from dishes.
Transportation rules
There are special GOSTs that stipulate the rules for transporting phosphoric acid, which is classified as dangerous goods. The substance can be delivered by any type of transport. The chemically active liquid is transported in tightly closed:
- steel tank trucks;
- bottles made of polyethylene, glass;
- plastic cubes;
- barrels;
- cans;
- rubberized railway tanks.
Price
Phosphoric acid can be purchased in pharmacies, hardware stores, and ordered through Internet sites. For industrial purposes, they are purchased in bulk at discounts. The average cost for Moscow in rubles is:
| Quantity, liter | Average price, rub. | |
| Food thermal | 1 | 400 |
| Technical 85% | 0,8 | 380 |
| 1600 | 13500 | |
| Soldering flux | 0,01 | 180 |
| 0,003 | 40 | |
| Food additive E388 | 1 | 85 |