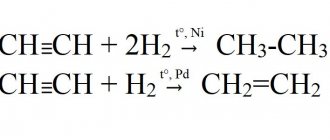Methane CH4
is a saturated hydrocarbon containing one carbon atom in the carbon chain. A colorless gas, tasteless and odorless, lighter than water, insoluble in water and does not mix with it.
All alkanes are substances that are similar in physical and chemical properties and differ by one or more –CH2– groups from each other. Such substances are called homologues , and a series of substances that are homologues is called a homologous series .
The very first representative of the homologous series of alkanes is methane CH4, or H–CH2–H.
The homologous series can be continued by sequentially adding a –CH2– group to the hydrocarbon chain of the alkane.
| Alkane name | Alkane formula |
| Methane | CH4 |
| Ethane | C2H6 |
| Propane | C3H8 |
| Butane | C4H10 |
| Pentane | C5H12 |
| Hexane | C6H14 |
| Heptane | C7H16 |
| Octane | C8H18 |
| Nonan | C9H20 |
| Dean | C10H22 |
The general formula of the homologous series of alkanes is CnH2n+2.
The first four members of the homologous series of alkanes are gases, C5–C17 are liquids, and starting from C18 are solids.
There are C–H bonds in the methane molecule. The C–H bond is covalent and weakly polar. This is a single σ bond. The carbon atom in methane forms four σ bonds. Consequently, the hybridization of the carbon atom in the methane molecule is sp3:
When a C–H bond is formed, the sp3-hybrid orbital of the carbon atom and the s-orbital of the hydrogen atom overlap:
The four sp3 hybrid orbitals of the carbon atom repel each other and are arranged in space so that the angle between the orbitals is the maximum possible.
Therefore, the four hybrid orbitals of carbon in alkanes are directed in space at an angle of 109° 28′ to each other:
This corresponds to the tetrahedral structure of the molecule.
| For example, in a CH4 methane molecule, hydrogen atoms are located in space at the vertices of a tetrahedron, the center of which is a carbon atom |
Methane is not characterized by the presence of isomers – neither structural (isomerism of the carbon skeleton, position of substituents) nor spatial.
Methane
is a saturated hydrocarbon, so it cannot enter into addition reactions.
Methane is characterized by the following reactions:
- decomposition,
- substitutions,
- oxidation.
The cleavage of weakly polar C–H bonds occurs only through a homolytic mechanism with the formation of free radicals.
Therefore, methane is characterized only by radical reactions.
Methane is resistant to strong oxidizing agents (KMnO4, K2Cr2O7, etc.) and does not react with concentrated acids, alkalis, or bromine water.
Substitution reactions
Methane is characterized by radical substitution reactions.
1.1. Halogenation
Methane reacts with chlorine and bromine in light or heat .
When methane is chlorinated, chloromethane is first formed:
Chloromethane can react with chlorine further to form dichloromethane, trichloromethane and carbon tetrachloride:
| The chemical activity of chlorine is higher than that of bromine, so chlorination occurs quickly and indiscriminately. |
Bromination occurs more slowly .
Substitution reactions in alkanes proceed via a free radical mechanism.
Free radicals R∙ are atoms or groups of interconnected atoms that contain an unpaired electron.
First stage. Initiating a chain.
Under the influence of a quantum of light or upon heating, the halogen molecule breaks into two radicals:
Free radicals are very active particles that strive to form a bond with some other atom.
Second stage. Development of the chain.
A halogen radical interacts with an alkane molecule and abstracts hydrogen from it.
In this case, an intermediate particle is formed - an alkyl radical, which in turn interacts with a new undisintegrated chlorine molecule:
Third stage. Circuit break.
When a chain process occurs, sooner or later radicals collide with radicals, forming molecules, and the radical process ends.
Both the same and different radicals can collide, including two methyl radicals:
1.2. Methane nitration
Methane reacts with dilute nitric acid by a radical mechanism when heated to 140°C and under pressure. The hydrogen atom in methane is replaced by the nitro group NO2.
| For example. When methane is nitrated, nitromethane is formed predominantly: CH4 + HNO3 = CH3NO2 + H2O |
Analysis of the radical reaction of plasma pyrolysis of methane"
Author — tokarna***@rambler.ru
CONTENT
INTRODUCTION………………………………………………………………………………3
1 Methods for producing acetylene from methane……………………………4
1.1 Physico-chemical properties of substances - participants in the reaction………5
2 Mechanism of hydrogen plasma formation……………………………7
3 Mechanism of the reaction of methane pyrolysis in a jet of hydrogen plasma…….7
4 Study of the dependence of changes in the concentrations of methane decomposition products, temperature and speed of the plasma jet on time………………………………………………………………………………………..9
CONCLUSIONS………………………………………………………………………………….12
LIST OF SOURCES USED…………………..13
INTRODUCTION
Modern chemical technology is distinguished by the desire to use increasingly higher operating parameters (temperature, pressure, flow rates of reacting substances), which make it possible to change the passage of chemical reactions in the desired direction. The use of low-temperature plasma opens up broad prospects in this regard.
Pyrolysis of carbon-containing compounds in the plasma of reducing and inert gases is attractive from the point of view of the possibility of obtaining unsaturated compounds and, first of all, acetylene, as well as the possibility of plasma production of high-quality soot.
Almost replaced by ethylene and propylene in the 60s of the last century, acetylene as a chemical raw material has not lost its importance in organic synthesis due to changes in the structure of the fuel balance. Currently, attention to acetylene has again increased. Acetylene is a starting product in the production of vinyl acetate, vinyl chloride, acrylonitrile and other monomers with their subsequent conversion into polymers. The most noticeable increase in acetylene consumption for the synthesis of 1,4-butanediol. In addition, acetylene is quite widely used in welding work (the so-called “balloon” acetylene).
The purpose of this work is to analyze the reaction of methane pyrolysis in a low-temperature plasma flow.
The main objectives of the analysis are to study the properties of all participants in the reaction, analyze the mechanism of the radical reaction and study the dependence of changes in the concentrations of methane decomposition products, temperature and speed of the plasma jet on time.
1 Methods for producing acetylene from methane
The most common method for producing acetylene is the oxidative pyrolysis of natural gas or naphtha (low-octane gasoline) at temperatures of 1600-1800 K due to exothermic reactions of hydrocarbon oxidation with oxygen. This process significantly replaces the cumbersome, environmentally harmful and energy-intensive carbide method. However, a comparison of oxidative pyrolysis with plasma-chemical pyrolysis in terms of main indicators demonstrates the advantages of the latter (Table 1).
Table 1 - Comparison of oxidative pyrolysis with plasma-chemical pyrolysis according to the main indicators
| Indicators | Oxidative pyrolysis | Plasma-chemical pyrolysis |
| Consumption of CH4 (t) per 1 t of C2H2 | 7,5 — 8 | 1,7 |
| Selectivity of CH4 to C2H2, % | 30 | 65 — 85 |
| Concentration of C2H2 in pyrolysis gas, % vol. | 10 | 12 — 25 |
| Energy intensity, t.e.* 1 t C2H2 | 11,8 | 6,3 |
*here. – tons of standard fuel
As can be seen from Table 1, the concentration of acetylene in the products of oxidative pyrolysis is two times less than in the plasma-chemical method, the consumption of raw materials per 1 ton of acetylene is almost 2.5 - 3 times higher in the oxidative method compared to the plasma-chemical method, energy intensity (consumption of all types of resources , expressed in t.u.t.) of the plasma-chemical method is almost two times (80%) lower than the oxidative method, the cost of acetylene obtained by the plasma-chemical method is 40% lower compared to the oxidative method. In addition, the plasma-chemical method for producing acetylene does not require the construction of an oxygen plant, which is necessary for the oxidative method.
When choosing one or another method for producing acetylene, it is necessary to assess the raw material base, related industries, and the specifics of the region.
The raw materials in oxidative production are: 1) hydrocarbons; 2) oxygen; 3) water vapor. The by-products of this process—synthesis gas and nitrogen—require their consumers.
The plasma-chemical method requires: 1) a raw material base - carbon-containing substances of various origins and electricity; 2) in the consumer of by-products - hydrogen and soot.
Currently, ethylene production also faces a number of problems with traditional methods of producing it in tube furnaces. The raw materials in these processes are light fractions of petroleum products. However, due to changes in the structure of consumption of petroleum products and increased demand for motor fuel, the main raw materials for the production of ethylene are fuel oil and other heavy fractions, the use of which in traditional technologies is very difficult due to the significant yield of tars and carbon. This also stimulates the introduction of plasma-chemical pyrolysis methods.
The undemanding nature of raw materials—one of the advantages of plasma-chemical processes—gives an additional advantage to this method. A wide range of carbon-containing materials, from methane to coal, were used as raw materials in plasma-chemical pyrolysis to produce acetylene.
1.1 Physicochemical properties of substances participating in the reaction
The main physicochemical properties of the substances participating in the reaction are given in Table 2.
Table 2 - Physico-chemical properties of substances participating in the reaction [3]
| Substance | Melting point, ℃ | Boiling point, ℃ | Application | Toxic effect |
| 1 | 2 | 3 | 4 | 5 |
| CH4 (methane) | -182,5 | -161,58 | As a fuel, for the production of water gas, hydrogen, acetylene, in the production of soot, methyl chloride, hydrocyanic acid. | The first signs of asphyxia (increased heart rate, increased breathing volume, etc.) begin to appear when the oxygen content in the air drops by 25-30%; a mixture of 80% methane and 20% oxygen causes headaches. Hazard class – 4. |
| NS CH (acetylene) | -80,0 | 83,8 | For autogenous welding, for lighting purposes; as a starting product for the production of acetaldehyde, vinyl ethers, vinyl chloride, tetrachloroethane, acrylic acid nitrile, etc. | Strong excitement, followed by a coma, cyanosis, immobility of the pupils, weak and irregular pulse. Hazard class – 4. |
| H2C=CH2 (ethylene) | -169,2 | -103,7 | Raw materials in the production of polyethylene, ethylene oxide, ethyl alcohol, ethanolamines, PVC, thiokol, etc. | The odor is felt at a concentration of 0.02 – 0.026 mg/l. Rapid anesthesia without a noticeable stage of excitation occurs at 80% ethylene with O2. Hazard class – 4. |
| H3C-CH3 (ethane) | -182,8 | -89 | The main use of ethane in industry is the production of ethylene. | Low toxic. Has a narcotic effect. Hazard class – 4. |
| C (soot) | — | 360 – 380 (temperature ignition) | In the production of rubber and printing industry as a dye. | Hazard class – 4. |
| H2 (hydrogen) | -259,14 | -252,8 | For the synthesis of ammonia, urea, methanol; during the hydrogenation of fats, petroleum products, coals and resins; as a reducing agent; for autogenous cutting and welding; for filling balloons, meteorological balloons. | Physiologically inert gas. Causes asphyxiation in very high concentrations due to a decrease in normal oxygen pressure. The narcotic effect occurs at very high pressures. Hazard class – 2. |
All substances involved in the reaction are not highly toxic substances and are widely used in both the chemical and petrochemical industries.
2 Mechanism of formation of hydrogen plasma
A gas, hydrogen, enters the plasmatron between two electrodes. An electric current passes through the electrodes (one is graphite, the other is ferrite). Under the influence of electric current, a molecular hydrogen ion and an electron are formed according to the following scheme:
The resulting electron is transferred to the metal surface.
A molecular hydrogen ion under the influence of an electric current dissociates into a hydrogen ion and a hydrogen atom:
Then the ionization of the hydrogen atom occurs:
This creates a partially ionized gas containing neutral atoms (molecules) and charged particles (ions and electrons).
3 Mechanism of the reaction of methane pyrolysis in a jet of hydrogen plasma
The main type of hydrocarbon raw material for the production of acetylene is natural gas - methane. The reaction takes place at 1500 - 1600 and at atmospheric pressure in a jet of hydrogen plasma, which is a high-temperature coolant.
The main difficulty in producing acetylene by pyrolysis of natural gas is the need to create high temperatures and supply large amounts of heat to the endothermic reaction of acetylene formation from methane.
In general, the reaction for producing acetylene from methane and its molar balance in the plasma flow has the form:
CH4 HC CH + H2C=CH2 + H3C-CH3 + C + CH4 + H2 – 90
1 0,60 0,21 0,05 0,01 0,06 0,77
The temperature of the plasma jet at the beginning of the process drops very quickly due to the transfer of heat to the introduced methane and the occurrence of endothermic reactions of its decomposition.
The formation of acetylene from methane occurs according to the mechanism proposed by Cassel. Active hydrogen atoms that are part of the plasma abstract hydrogen from the methane molecule, forming the corresponding radicals:
It is easier to remove hydrogen from an ethylene molecule, since the C-C bond energy in an ethylene molecule is 101.16, and the C-H bond energy is 85.56.
Open circuit stage:
The thermodynamic stability of acetylene at temperatures above 1100 K is greater than that of other hydrocarbons. However, at very high temperatures there are competitive reactions of compaction and decomposition of acetylene [6, p.64].
NS CH C + H2 + 54.9
In this regard, pyrolysis to produce acetylene is carried out at very short contact times with rapid “hardening” of the reaction products.
4 Study of the dependence of changes in the concentrations of methane decomposition products, temperature and speed of the plasma jet on time
An important prerequisite for the scientific development of the plasma-chemical process of methane pyrolysis is the calculation of the kinetics of this process. A system of equations of hydrodynamics and chemical kinetics of a high-temperature gas jet in which methane decomposes and acetylene is “quenched” was numerically integrated on an electronic computer. The equations of chemical kinetics were written based on the assumption of the formation of acetylene from methane according to the well-known Kassel scheme. The results of solving this system of equations are shown in Figures 1, 2, 3. [1, p.283].
Figure 1 – Dependence of changes in the concentrations of methane decomposition products, temperature and plasma jet speed on time
As can be seen from Figure 1, in the first stages of the reaction there is a rapid decrease in temperature. This decrease in temperature is explained by the occurrence of a mainly endothermic reaction of methane decomposition, which occurs at a very high rate at the initial reaction temperature.
Figure 2 – Dependence of changes in the concentrations of methane decomposition products, temperature and velocity of the plasma jet on time
As can be seen from Figure 2, as the temperature decreases, the rate of methane decomposition drops sharply, and spontaneous inhibition of the reaction occurs, which can be called auto-hardening, in contrast to forced hardening, carried out by special external influences.
Figure 3 – Dependence of changes in the concentrations of methane decomposition products, temperature and velocity of the plasma jet on time
As can be seen from Figure 3, at further stages of the reaction, acetylene decomposes to soot and hydrogen, accompanied by the release of heat and an increase in temperature. To eliminate unwanted decomposition of acetylene, it is necessary to carry out forced hardening. The place, time and rate of forced hardening are determined by the kinetics of the processes under consideration. Kinetic calculations made it possible to estimate the rate of forced temperature decrease required to preserve the formed acetylene. It is ~ 106 degrees/sec.
Since the pyrolysis of methane in a plasma jet of hydrogen may be of industrial interest, hydrogen was also used as a plasma-forming gas in the laboratory setup. The choice of hydrogen as a plasma-forming substance is primarily due to the fact that hydrogen is one of the main reaction products, the volumetric yield of which is three times higher than the volumetric yield of acetylene. Consequently, under industrial production conditions, the process of methane pyrolysis in a hydrogen plasma jet is abundantly supplied with hydrogen as a working fluid for the plasma torch. In addition, at a temperature of about 4500-5000 K, hydrogen, almost completely dissociated into atoms, is an effective coolant and reagent. It also prevents the formation of soot and, in addition, has a significant effect on the chemistry of the process.
CONCLUSIONS
- The reaction of plasma pyrolysis of methane, the target product of which is acetylene, has been studied. However, at temperatures above 1100 K, competitive reactions of acetylene compaction and decomposition occur, and therefore pyrolysis is carried out with rapid “hardening” of the reaction products.
- The dependence of changes in the concentrations of methane decomposition products, the temperature and speed of the plasma jet on time has been studied and it has been established that the temperature of the plasma jet at the beginning of the process drops very quickly due to the transfer of heat to the introduced methane and the occurrence of endothermic reactions of its decomposition. At the end of the process, the temperature rises slightly due to the release of heat as acetylene begins to decompose into carbon and hydrogen.
Methane oxidation
Alkanes are low-polar compounds, so under normal conditions they are not oxidized even by strong oxidizing agents (potassium permanganate, potassium chromate or dichromate, etc.).
3.1. Complete oxidation - combustion
Alkanes burn to form carbon dioxide and water. The combustion reaction of alkanes is accompanied by the release of a large amount of heat.
CH4 + 2O2 → CO2 + 2H2O + Q
The general equation for the combustion of alkanes is:
CnH2n+2 + (3n+1)/2O2 → nCO2 + (n+1)H2O + Q
When alkanes burn in a lack of oxygen, carbon monoxide CO or soot C can be formed.
Of industrial importance is the reaction of methane oxidation with oxygen to a simple substance - carbon:
CH4 + O2 → C + 2H2O
This reaction is used to produce soot.
3.2. Catalytic oxidation
- During the catalytic oxidation of methane with oxygen, the formation of various products is possible depending on the conditions of the process and the catalyst. Possible formation of methanol, formic aldehyde or formic acid:
- Steam reforming of methane is important in industry: the oxidation of methane with water vapor at high temperature.
The reaction product is the so-called “synthesis gas”.
Method for producing acetylene from methane
Method for producing acetylene from methane
Illustrations
show all
The invention relates to a method for producing acetylene by oxidative pyrolysis of methane in the presence of oxygen and a catalyst, characterized in that the catalyst is heated by passing an electric current through it to temperatures of 700-1200°C; a fechral alloy heat-treated in air at temperatures of 900-1100°C is used as a catalyst. , and the methane:oxygen ratio is changed in the range of 5:1-15:1. The use of this method makes it possible to increase the yield and selectivity of the process. 1 salary files, 1 table, 1 ill.
Essay
The invention relates to the field of oil refining, petrochemical industry and gas processing, namely to a method for producing acetylene from methane.
As is known, there is still no acceptable technology for using associated and petroleum gases, which are flared in tens of millions of tons per year. There are several directions for the development of technologies for the utilization of this hydrocarbon raw material, in which research is being carried out. This is, for example, the decomposition of C1-C3 paraffins to produce hydrogen as the main target product. Another route is to convert natural gas into chemical products, which relies on a complex energy- and capital-intensive process of pre-conversion into synthesis gas.
Recently, there has been interest in the one-step process of converting methane into chemical products, for example, high-temperature (≥900°C) oxidative pyrolysis of C1-C3 gases to acetylene. The fact of acetylene formation during incomplete combustion of hydrocarbons has been known for a long time (Evlanov S.F., Lavrov N.V. // Scientific principles of catalytic conversion of hydrocarbons, 1977, pp. 210-232). When optimizing the yield of acetylene, emphasis was placed on the design of various types of burners and the selection of various conditions for the combustion of hydrocarbon raw materials. The process of oxidative pyrolysis has a number of features: a very short duration of presence of the starting substances in the reactor (several milliseconds), high reaction temperature (1200-1400°C), pressure - atmospheric or slightly increased, sharp cooling of the reaction products to a temperature below 300°C using as a cooling medium of water or oil (sharp cooling prevents the decomposition of the resulting acetylene into carbon and hydrogen).
Patent EP No. 0178853 describes a technology according to which part of the methane is partially burned, and a high-temperature gas mixture (T>1000°C) is passed through a fluidized or gushing layer of particles of inert material: fireclay, quartz, corundum, zirconium dioxide, carborundum, etc. for reducing the temperature gradient in the gas flow. In this case, the maximum selectivity and yield for C2+ hydrocarbons are 30% with a methane conversion of 62.8% at 1151°C.
The disadvantage of this method is that part of the methane is consumed as fuel to heat the entire mass of gas to 1200-1400°C. In addition, to preserve C2+ hydrocarbon molecules that are less durable than methane, the entire mixture is quenched with water, i.e. rapid cooling of the mixture to ~300°C, which greatly complicates the technology.
There are known works on the study of oxidative condensation of methane, in which the main attention was paid to the search for catalysts and the study of the mechanism of the reaction occurring at 650-850 ° C (V.S. Arutyunov, O.V. Krylov. Oxidative transformations of methane. M.: Nauka, 1998 ). It is at these temperatures (650-850°C) that it is possible to obtain significant yields of ethane and ethylene from the oxidation of methane.
The closest to the proposed method is the method for producing C2-C4 hydrocarbons, where the term “catalytic oxidative pyrolysis of methane” was used for the first time (A.S. USSR No. 1216937, prototype). This method is carried out at 800-950°C, contact time 0.1-2.5 s, content in the initial mixture of 10-20 vol.% oxygen and 90-80 vol.% methane in the presence of a catalyst composition, mol.%: Bi2O3 0.95-7.59 and MeO 92.41-99.05, where Me is Mg, Ca, Sr, Ba. The catalyst made it possible to increase the yield of C2+ hydrocarbons to 10 mol.% per passed methane with a selectivity of 78.5%. However, the main products are ethane and ethylene. There is no acetylene in the resulting mixture, and the methane conversion is very low - up to 20%, which gives low product yields.
The purpose of this invention is to increase the yield and selectivity of the process for acetylene through the use of a new type of catalyst and changes in the technology of oxidative pyrolysis.
The proposed method for producing acetylene by oxidative pyrolysis of methane in the presence of oxygen involves the use of a fechral alloy in the form of spirals, tapes, rods and other forms as a catalyst, heat-treated in air at 900-1100°C. The catalyst is heated by passing an electric current through it to temperatures of 700-1200°C, and the methane: oxygen ratio is changed in the range of 5:1-15:1.
Contact of oxygen-containing mixtures based on methane in certain proportions of CH4/O2 (air) with a wire made of heat-treated fechral, heated and electric current to temperatures from 750°C to >1200°C gives a catalytic effect with a change in selectivity for C2-hydrocarbons compared to the gas phase , oxidative pyrolysis of methane. The gas mixture is supplied cold, and only contact with the hot fechral alloy leads to a catalytic effect. Since the gas mixture is supplied cold, the breakthrough of part of the cold gas leads to a sharp cooling of the reaction products formed upon contact with high-temperature fechral, i.e. to hardening the products of oxidative pyrolysis and increasing the yield of C2 hydrocarbons, primarily acetylene.
The Fechral alloy was used as a catalyst in various forms, mainly in the form of wire with a diameter of 0.25 mm.
Alloy grade: X 23 Yu5 T, composition: C - up to 0.05%; Si - up to 0.5%; Mn - up to 0.3%; Ni - up to 0.6%; S - up to 0.015%; P - up to 0.03%; Cr - 22-24% Ce - up to 0.1%; Ti - 0.2-0.5%; Al - 5-5.8%; Ca - up to 0.1%; the rest is iron. Manufacturer: JSC Metallurgical, GOST 12766 1-90.
The preparation of fechral included several operations: a wire or tape weighing 0.26-0.28 g was twisted into a spiral with an outer diameter of 5 mm, degreased by washing in acetone and calcined in air in a muffle furnace at a temperature of 1000°C for 21 hours. As a result of such oxidative heat treatment, an oxide layer is formed on the surface of the alloy, consisting mainly of aluminum oxide.
The spiral prepared in this way was put on a ceramic tube with a diameter of 2 mm and placed in a flow reactor.
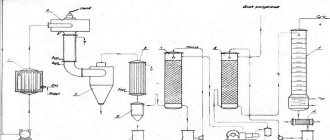
The installation diagram for the oxidative pyrolysis of methane is shown in the drawing: a - front view, b - side view. The installation diagram includes a quartz reactor 1, a fechral spiral 2 on a ceramic tube 3, a laboratory autotransformer (LATR) 4, an optical quartz window 5 and an optical pyrometer 6 with laser guidance on the spiral.
The reactor works as follows. Voltage is applied to spiral 1 using LATR 3, which allows it to be heated to the required temperatures from 700 to 1200°C. The temperature is recorded using an optical pyrometer 6 (PD-7, Omsk) with laser guidance on the spiral. For this purpose, the reactor is equipped with an optical quartz window 5. The initial reaction gas is supplied to the heated wire. The gas comes into contact with the heated coil and exits. A sample of the mixture after the reactor is sent for chromatographic analysis, which is carried out on a TsVET-500M chromatograph. The content of residual methane, as well as the resulting ethane, ethylene and acetylene, is recorded using a flame ionization detector. Chromatography conditions: capillary column with a stationary phase of SiO2 15 m long, carrier gas pressure - nitrogen - 1 kgf/cm2, air flow 300 ml/min, hydrogen flow 30 ml/min, column temperature 50°C.
The proposed method is illustrated with examples.
Example 1.
A reaction mixture of the composition: 15 vol.% methane and 85 vol.% nitrogen (without oxygen, methane/oxygen ratio 1/0) is passed through the reactor at a spiral temperature of 1100°C with a volumetric flow rate of 76 ml/min. The spiral is made of wire with a diameter of 0.25 mm. Methane conversion and product selectivity are given in the table.
Example 2-3.
Similar to example 1, but the reaction mixture is passed through the reactor at a spiral temperature of 760°C and 890°C, respectively. The spiral is made of tape 1 mm wide and 0.4 mm thick. Methane conversion and product selectivity are given in the table.
Example 4.
Similar to example 1, but the reaction mixture is passed through the reactor at a spiral temperature of 970°C. Methane conversion and product selectivity are given in the table.
Example 5.
Similar to example 1, but the reaction mixture of the composition: 15 vol.% methane, 1 vol.% oxygen (methane/oxygen ratio 15/1) and 84 vol.% nitrogen was passed through the reactor at a spiral temperature of 1170°C.
Example 6 and 7.
Similar to example 5, but the reaction mixture is passed through the reactor at a spiral temperature of 1000°C and 960°C.
Example 8.
Similar to example 5, but the reaction mixture is passed through the reactor at a spiral temperature of 830°C. The spiral is made of tape 1 mm wide and 0.2 mm thick.
Example 9.
Similar to example 5, but the reaction mixture is passed through the reactor at a wire temperature of 750°C.
Example 10.
A reaction mixture of the composition: 15 vol.% methane, 1 vol.% oxygen (methane/oxygen ratio 15/1) and 84 vol.% nitrogen is passed through the reactor at a coil temperature of 1160°C and a flow rate through the reactor of 80 ml/min.
Example 11.
Similar to example 10, but the reaction mixture of the composition: 20 vol.% methane, 1.33 vol.% oxygen (methane/oxygen ratio 15/1) and 78.67 vol.% nitrogen is passed through the reactor at a spiral temperature of 1150°C.
Example 12.
A reaction mixture of the composition: 15 vol.% methane, 1.67 vol.% oxygen (methane/oxygen ratio 9/1) and 83.33 vol.% nitrogen is passed through the reactor at a wire temperature of 760°C. Flow rate 75 ml/min. The spiral is made of wire with a diameter of 0.25 mm.
Example 13 and 14.
Similar to example 12, but the reaction mixture is passed through the reactor at a wire temperature of 850°C and 930°C, respectively. The spiral is made of tape 1 mm wide and 0.4 mm thick.
Example 15, 16 and 17.
Similar to example 12, but the reaction mixture is passed through the reactor at wire temperatures of 1040°C, 1110°C and 1230°C, respectively.
Methane conversion and product selectivity are given in the table. As can be seen from the table, this invention makes it possible to obtain acetylene with a selectivity of up to 41.8% and ethylene with a selectivity of 3.3%, methane conversion of 56.4%.
The total yield of C2 hydrocarbons has been significantly increased - 25.5% (the maximum for the prototype is 9.7%).
1. A method for producing acetylene by oxidative pyrolysis of methane in the presence of oxygen and a catalyst, characterized in that the catalyst is heated by passing an electric current through it to temperatures of 700-1200°C, a fechral alloy heat-treated in air at temperatures of 900-1100°C is used as a catalyst, and the methane:oxygen ratio is changed in the range of 5:1-15:1.
2. The method according to claim 1, characterized in that the heat-treated fechral alloy is used in the form of spirals, tapes, rods.
Decarboxylation of carboxylic acid salts (Dumas reaction)
The Dumas reaction is the interaction of salts of carboxylic acids with alkalis during fusion.
R–COONa + NaOH → R–H + Na 2 CO 3
Decarboxylation is the removal (elimination) of a carbon dioxide molecule from a carboxyl group (-COOH) or an organic acid or carboxylate group (-COOMe) of a salt of an organic acid.
When sodium acetate reacts with sodium hydroxide during fusion, methane and sodium carbonate are formed:
Training tasks
1. For methane, the following statements are true:
1) its molecule is formed by a carbon atom in the sp-hybrid state 2) it is a low-boiling liquid, highly soluble in water 3) it is a low-boiling gas, poorly soluble in water 4) is the main component of natural gas 5) easily reacts with dilute sulfuric acid
2. For methane, the following statements are true:
1) its molecule is formed by a carbon atom in a state of sp2 hybridization 2) methane reacts with vapors of dilute nitric acid 3) methane has a characteristic unpleasant odor 4) burns in air to form carbon monoxide and water 5) burns in air to form carbon dioxide and water .
3. For ethane the following statements are true:
1) it is a colorless gas, slightly lighter than air 2) it is a colorless gas, slightly heavier than air 3) when it reacts with water, ethyl alcohol is formed 4) when it is dehydrogenated, ethylene is formed 5) all the carbon atoms in it are tertiary
4. For ethane the following statements are true:
1) both carbon atoms in its molecule are primary 2) does not react with sodium hydroxide 3) reacts with sulfuric acid 4) reacts with methane 5) has a strong unpleasant odor
5. For ethylene the following statements are true:
1) both carbon atoms in its molecule are in a state of sp2 hybridization 2) the density of ethylene vapor is equal to the density of nitrogen vapor 3) does not react with water 4) does not burn in oxygen 5) does not add chlorine
6. For ethylene the following statements are true:
1) under normal conditions it is a low-boiling liquid, highly soluble in water 2) both carbon atoms in its molecule are in a state of sp3 hybridization 3) reacts with water to form acetic acid 4) reacts with bromine water to form 1,2-dibromoethane 5 ) reacts with water to form ethyl alcohol
7. For acetylene the following statements are true:
1) under normal conditions it is a gas whose vapors are lighter than air 2) under normal conditions it is a gas whose vapors are heavier than air 3) does not react with bromine 4) reacts with water to form ethanol 5) reacts with water to form acetaldehyde
8. For acetylene the following statements are true:
1) the carbon atoms in its molecule are in a state of sp2 hybridization and are connected by a double bond 2) the carbon atoms in its molecule are connected by a triple bond and are in a state of sp hybridization 3) when it burns in oxygen, carbon monoxide and water are formed 4) when it combustion in oxygen produces carbon dioxide and water 5) reacts with nitrogen