Alkynes are unsaturated (unsaturated) non-cyclic hydrocarbons, the molecules of which contain one triple bond between the carbon atoms C≡C.
Let us dwell on the properties, methods of preparation and structural features of alkynes.
Structure, isomerism and homologous series of alkynes
Chemical properties of alkynes
Preparation of alkynes
Alkynes are unsaturated hydrocarbons whose molecules contain one triple bond. The structure and properties of the triple bond determine the characteristic chemical properties of alkynes. The chemical properties of alkynes are similar to the chemical properties of alkenes due to the presence of a multiple bond in the molecule.
Alkynes are characterized by oxidation reactions. The oxidation of alkenes occurs predominantly at the triple bond, although hard oxidation (combustion) is also possible.
Receipt
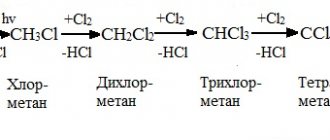
All methods for the industrial production of acetylene converge into two types: hydrolysis of calcium carbide and pyrolysis of various hydrocarbons. The latter requires less energy, but the purity of the product is quite low. The carbide method is the opposite.
The essence of pyrolysis is that methane, ethane or other light hydrocarbon, when heated to high temperatures (from 1000 °C), is converted into acetylene with the release of hydrogen. Heating can be carried out by electric discharge, plasma or combustion of part of the raw material. But the problem is that as a result of the pyrolysis reaction, not only acetylene can be formed, but also many different products that must subsequently be disposed of.
The carbide method is based on the reaction of calcium carbide with water. Calcium carbide is produced from its oxide by fusing with coke in electric furnaces. Hence the high energy consumption. But the purity of acetylene obtained in this way is extremely high (99.9%).
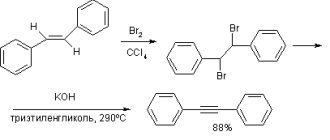
In the laboratory, acetylene can also be obtained by dehydrohalogenation of dihalogenated alkanes using an alcoholic alkali solution.
Chemical properties of acetylene
Based on the triple bond of acetylene, it will be characterized by addition reactions and polymerization reactions. Hydrogen atoms in an acetylene molecule can be replaced by other atoms or groups. Therefore, we can say that acetylene exhibits acidic properties. Let's look at the chemical properties of acetylene based on specific reactions.
- Hydrogenation. It is carried out at high temperature and in the presence of a catalyst (Ni, Pt, Pd). On a palladium catalyst, incomplete hydrogenation is possible.
- Halogenation. It can be either partial or complete. Goes easily even without catalysts or heat. In the light, chlorination occurs explosively. In this case, acetylene completely decomposes to carbon.
- Addition to acetic acid and ethyl alcohol. Reactions take place only in the presence of catalysts.
- Addition of hydrocyanic acid.
- Interaction of acetylene with metal-organic compounds.
- Interaction with sodium metal. A temperature of 150 °C or preliminary dissolution of sodium in ammonia is required.
2CH≡CH + 2Na → 2CH≡CNa + H2
- Interaction with complex salts of copper and silver.
- Interaction with sodium amide.
- Dimerization. In this reaction, two acetylene molecules combine into one. A catalyst is required - a cuprous salt.
- Trimerization. In this reaction, three acetylene molecules form benzene. Requires heating to 70 °C, pressure and a catalyst.
- Tetramerization. As a result of the reaction, an eight-membered ring is obtained - cyclooctatetraene. This reaction also requires little heat, pressure and an appropriate catalyst. Typically these are complex compounds of divalent nickel.
These are not all the chemical properties of acetylene.
General information
Acetylene is an unsaturated hydrocarbon C 2 H 2 . It has a triple bond between carbon atoms and belongs to the class of alkynes. It is practically not found in nature on Earth, because Due to the presence of oxygen, this extremely unstable compound is obtained by synthesis. Acetylene has been found in the atmospheres of Uranus, Jupiter and Saturn.
Read also: How to remove the chuck of an assault screwdriver
Gaseous acetylene was first obtained in 1836 by Edmund Davy during the decomposition of potassium carbide with water, obtained by fusing potassium metal with coal: K 2 C 2 + 2 H 2 O = C 2 H 2 + 2 KOH.
Since the end of the 19th century, when a cheap method was developed for producing acetylene from calcium carbide (CaC 2 + 2H 2 O = C 2 H 2 + Ca(OH) 2, which in turn was obtained by calcining a mixture of coal and quicklime (CaO + 3C = CaC 2 + CO), this gas began to be used for lighting. In a flame at high temperature, acetylene, containing 92.3% carbon (this is a kind of chemical record), decomposes to form solid carbon particles, which can contain from several to millions of carbon atoms. Strongly heated in the inner cone of the flame, these particles cause a bright glow of the flame - from yellow to white, depending on the temperature (the hotter the flame, the closer to white its color). Acetylene torches gave 15 times more light than ordinary gas lamps that illuminated the streets.Gradually they were replaced by electric lighting, but for a long time they were used in small lamps on bicycles, motorcycles, and horse-drawn carriages.
Application
The structural formula of acetylene shows us a fairly strong bond between carbon atoms. When it ruptures, for example during combustion, a lot of energy is released. For this reason, the acetylene flame has a record high temperature - about 4000 °C. It is used in torches for welding and cutting metal, as well as in rocket engines.
The acetylene combustion flame also has a very high brightness, so it is often used in lighting devices. It is also used in explosives. True, it is not acetylene itself that is used, but its salts.
As can be seen from its varied chemical properties, acetylene can be used as a raw material for the synthesis of other important substances: solvents, varnishes, polymers, synthetic fibers, plastics, organic glass, explosives and acetic acid.
Safety
As already mentioned, acetylene is a flammable substance. With oxygen or air it is capable of forming extremely flammable mixtures. A single spark from static electricity, heating up to 500 °C or slight pressure is enough to cause an explosion. At a temperature of 335 °C, pure acetylene spontaneously ignites.
Because of this, acetylene is stored in pressure cylinders that are filled with a porous substance (pumice, activated carbon, asbestos). In this way, acetylene is distributed throughout the pores, reducing the risk of explosion. Often these pores are impregnated with acetone, which results in the formation of an acetylene solution. Sometimes acetylene is diluted with other, more inert gases (nitrogen, methane, propane).
This gas also has a toxic effect. When it is inhaled, intoxication of the body will begin. Signs of poisoning are nausea, vomiting, tinnitus, and dizziness. Large concentrations can even lead to loss of consciousness.
References
- CAS number
- Matheson Gas Handbook. "Lower and Upper Explosion Limits for Flammable Gases and Vapors (LEL/LEL)" (PDF) (English). Matheson Gas Products. paragraph 443. Consultado el October 2, 2016.
- Henry Enfield Roscoe and Karl Schorlemmer. A Treatise on Chemistry, D. Appleton and Co., 1833, p. 614, in which reference is made to the Reports of the British Association,
1836, p. 62. - “Because of the brightness with which the new gas burns on contact with the atmosphere, it is, in the opinion of the author, admirably adapted for artificial illumination if it can be obtained at a low cost.” William Joseph Dibdin. "Acetylene", Public Lighting by Gas and Electricity,
Ch. XXIX, p. 489. - American Council of Learned Societies. Dictionary of Scientific Biography,
Charles Scribner's Sons, New York, 1981, Vol. 2, p.67. - Popular illustrated encyclopedic dictionary Salvat (1906 - 1914)
Bibliography
- National Institute of Occupational Safety and Health of Spain: International Chemical Safety Data Sheet for Acetylene.
| authoritative control |
|
- Datos: Q133145
- Multimedia: Acetylene
Addition reactions
A triple bond consists of a σ bond and two π bonds. Let's compare the characteristics of a single C–C bond, a triple bond C ≡ C, and a C–H bond:
Thus, the C≡C triple bond is shorter than the C–C single bond, therefore the π-electrons of the triple bond are held more tightly by the nuclei of carbon atoms and have less polarizability and mobility. Triple bond addition reactions
Alkynes are characterized by addition reactions at the triple bond C ≡ C with breaking of π bonds.
1.1. Hydrogenation
The hydrogenation of alkynes occurs in the presence of catalysts (Ni, Pt) with the formation of alkenes, and then immediately alkanes.
When using a less active catalyst (Pd, CaCO3, Pb(CH3COO)2), hydrogenation stops at the stage of formation of alkenes.
1.2. Halogenation of alkynes
The addition of halogens to alkynes occurs even at room temperature in solution (solvents - water, CCl4).
Alkynes react similarly with chlorine, but chlorine water does not become discolored, because chlorine water is already colorless)
Reactions occur in the presence of polar solvents according to an ionic (electrophilic) mechanism.
1.3. Hydrohalogenation of alkynes
Alkynes add hydrogen halides. The reaction proceeds by the mechanism of electrophilic addition with the formation of a halogenated alkene or dihaloalkane.
When hydrogen halides and other polar molecules add to symmetrical alkynes, as a rule, one reaction product is formed, where both halogens are located at the same C atom.
When polar molecules add to unsymmetrical alkynes, a mixture of isomers is formed. In this case, Markovnikov's rule is satisfied.
1.4. Alkyne hydration
Hydration (addition of water) of alkynes occurs in the presence of an acid and a catalyst (mercury salt II).
First, an unstable alkene alcohol is formed, which then isomerizes to an aldehyde or ketone.
The hydration of alkynes occurs via an ionic (electrophilic) mechanism.
For unsymmetrical alkenes, the addition of water predominantly follows the Markovnikov rule.
1.5. Dimerization, trimerization and polymerization
The addition of one acetylene molecule to another ( dimerization ) occurs under the influence of an ammonia solution of copper (I) chloride. This produces vinyl acetylene:
Trimerization of acetylene (the addition of three molecules to each other) occurs under the influence of temperature, pressure and in the presence of activated carbon with the formation of benzene (Zelinsky reaction):
Alkynes also undergo polymerization - the process of repeatedly combining molecules of a low molecular weight substance (monomer) with each other to form a high molecular weight substance (polymer).
nM → Mn ( M is a monomer molecule)
… –CH=CH–CH=CH–CH=CH–…
Welding technology and modes
Acetylene-oxygen mixtures are used to join parts made of carbon and low-alloy steels. For example, this method is widely used to create permanent pipeline connections. For example, pipes with a diameter of 159 mm with a wall thickness of no more than 8 mm. But there are also some restrictions; joining steel grades 12×2M1, 12×2MFSR using this method is unacceptable.
Selecting mode parameters
To prepare the mixture necessary for combining metals, use the formula 1/1,2. When processing workpieces made of alloy steels, the welder must monitor the state of the flame. In particular, an excess of acetylene should not be allowed.
The consumption of the mixture with the oxygen/acetylene formula is 100-130 dm 3 /hour per 1 mm of thickness. The flame power is regulated using a burner, which is selected depending on the material used, its characteristics, thickness, etc.
To perform welding with acetylene, welding wire is used. Its grade must correspond to the steel grade of the parts being welded. The diameter of the wire is determined depending on the thickness of the metal being welded.
For the convenience of technologists and welders themselves, there are many tables on the basis of which you can quite easily select a welding mode. To do this you need to know the following parameters:
- wall thickness of welded workpieces;
- type of welding - left, right;
Read also: Connection diagram for a contactor via a time relay
Based on this, you can determine the diameter of the filler wire and select the acetylene consumption. For example, the thickness is 5-6 mm, tip No. 4 will be used to perform the work. That is, based on the tabular data, the wire diameter will be 3.5 mm for left welding, 3.5 mm for right welding. Acetylene consumption in this case will be for left welding method 60-780 dm 3 / hour, with the right 650-750 dm 3 / hour.
Welding is performed in small sections of 10-15 mm. The work is performed in the following sequence. At the first stage, the edges are melted. After this, the root suture is applied. Once the root formation is complete, welding can continue. If the thickness of the workpieces is 4 mm, then welding can be performed in one layer. If the thickness exceeds the specified one, then a second one must be applied. It is laid only after the root of the seam has been completed along the entire specified length.
To improve the quality of welding, preheating is allowed. That is, the future welded joint is heated using a torch. If this method is adopted as a basis, then warming up must be done again after each stop.
Seams can be made with gas in any spatial position. For example, when making a vertical seam there are some peculiarities. So, the vertical seam should be made from bottom to top.
When performing welding work, breaks in work are unacceptable, at least until the entire seam is cut. When stopping operation, the burner must be withdrawn slowly, otherwise seam defects - cavities and pores - may occur. An interesting feature exists when welding pipelines; a draft is not allowed in it and therefore the ends of the pipes must be plugged.
Alkyne oxidation
Oxidation reactions in organic chemistry are accompanied by an increase in the number of oxygen atoms (or the number of bonds with oxygen atoms) in the molecule and/or a decrease in the number of hydrogen atoms (or the number of bonds with hydrogen atoms).
2.1. Combustion of alkynes
Alkynes, like other hydrocarbons, burn to form carbon dioxide and water.
The general equation for the combustion of alkynes is:
2.2. Oxidation of alkynes with strong oxidizing agents
Alkynes react with strong oxidizing agents (permanganates or chromium (VI) compounds). In this case, oxidation of the C≡C triple bond and C-H bonds at carbon atoms at the triple bond . In this case, bonds with oxygen are formed.
When three bonds are oxidized at a carbon atom in an acidic environment, a carboxyl group COOH is formed, and four bonds form carbon dioxide CO2. In a neutral environment - a salt of a carboxylic acid and a carbonate (bicarbonate), respectively.
Correspondence table between the oxidized fragment of the molecule and the product:
When butine-2 is oxidized with potassium permanganate in sulfuric acid, two CH3–C ≡ fragments undergo oxidation, therefore acetic acid is formed:
When 3-methylpentine-1 is oxidized with potassium permanganate in sulfuric acid, the R–C and H–C fragments undergo oxidation, therefore carboxylic acid and carbon dioxide are formed:
When alkynes are oxidized by strong oxidizing agents in a neutral environment, carbon-containing products of the hard oxidation reaction (acid, carbon dioxide) can react with the alkali formed in the solution in a ratio determined by the electronic balance with the formation of the corresponding salts.
Similar organic products are formed when alkynes react with chromates or dichromates.
The oxidation of acetylene proceeds a little differently; the C–C σ bond is not broken, so oxalic acid is formed in an acidic environment:
In a neutral environment, a salt of oxalic acid is formed - potassium oxalate:
Discoloration of a solution of potassium permanganate is a qualitative reaction to a triple bond.