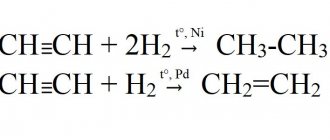Acetylene explosion
Since acetylene combustion and explosion are possible in the absence of oxidizing agents, including oxygen, it, like hydrogen, is the most explosive gas. The similarity with hydrogen is that they have the lowest ignition energy, but more on that later.
The explosiveness of flammable gases and vapors is characterized by an indicator - the amount of ignition energy. A substance with a low value is more explosive. Ignition energy values (MJ) for stoichiometric gas mixtures at atmospheric pressure and temperature 20°C are given in the table below.
| Gas | Mixture with air | Mixture with oxygen |
| Methane | 0,3 | 0,0038 |
| Ethane | 0,25 | 0,0019 |
| Propane | 0,24 | 0,002 |
| Hydrogen | 0,02 | 0,0003 |
| Acetylene | 0,019 | 0,0003 |
From the data in the table it can be seen that the ignition energy of mixtures with air is approximately 100 times greater than that of mixtures with oxygen.
Pure acetylene is capable of exploding upon rapid heating to 450-500°C and excess pressure over 1.5 kgf/cm2. The most explosive mixtures with air are those containing from 7 to 13% C2H2. If C2H2 is present in a mixture with air from 2.2 to 81% by volume, the mixture explodes at atmospheric pressure.
Also, at atmospheric pressure, a mixture of oxygen and acetylene from 2.8 to 93% by volume is explosive. The most explosive mixture with oxygen contains 30% C2H2. Therefore, strong local heating, a flame, and even a spark can cause an explosion of a mixture of acetylene with oxygen or air.
The explosiveness of pure acetylene is determined by pressure, temperature, and also depends on its purity, moisture content, the presence of catalysts, the nature of the explosion agent, the size and shape of the vessel, heat removal conditions and a number of other reasons. As the pressure increases, the molecules of acetylene gas come closer together, which facilitates the spread of decomposition throughout the entire mass of gas. This is confirmed, on the one hand, by the fact that liquid acetylene, in which the proximity of molecules is especially great, is a highly explosive substance even at ordinary temperatures. On the other hand, compressed acetylene loses its explosiveness if its molecules are in any way separated from each other. This is achieved by mixing acetylene with nitrogen or inert gases that do not interact with it, as well as by absorbing acetylene with acetone or other solvent in the presence of a porous substance. For example, wet acetylene is less explosive than dry acetylene. A mixture containing 1.15 volumes of C2H2 per volume of water vapor is not capable of explosive decomposition. This can be explained similarly to what was said above by the separation of C2H2 molecules by water vapor.
The explosion of acetylene or its mixture with oxygen and air is accompanied by the release of heat, resulting in an increase in pressure and temperature. Consequently, such explosions can cause destruction and accidents. Therefore, handling acetylene requires strict adherence to safety measures.
The pressure generated during an explosion depends on the initial parameters and nature of the explosion, and increases by approximately 10-15 times compared to the initial pressure
When acetylene is dissolved in liquids, its explosiveness decreases. It dissolves best in acetone, but more about this in the article on polymerization and dissolution of acetylene
With prolonged contact of C2H2 with copper, silver and mercury, explosive compounds are formed - acetylenoids. Acetylenoids explode on impact or when heated above 100°C. Therefore, for the manufacture of equipment in contact with acetylene, alloys with a copper content of no more than 70% are used.
That is why all equipment for transporting and storing acetylene is made of steel and has a specific design that excludes the possibility of connecting equipment for other gases.
The valve of the acetylene cylinder does not have a connecting thread and is opened with a specially designed socket wrench, and the reducer is connected with a clamp.
When chlorine reacts with acetylene containing even a small amount of air, an explosion occurs. Pure acetylene, when in contact with chlorine, explodes under intense light.
Basics of safe cylinder handling
Before installing the cylinder and connecting it to gas appliances, the first thing you need to do is make sure there is no damage, rust on the body and that the valve is working properly.
The main technical requirements that must be observed when operating cylinders include:
- All cylinders, with the exception of one (five-liter for connecting to a gas stove) must be installed in outbuildings outside buildings and at a distance no closer than 5 m from the entrance to them.
- Avoid storing cylinders in living rooms, basements and attics.
- Do not place cylinders closer than 1 m from heating devices and 5 m from open fire.
Obvious, but often forgotten safety measures when using vessels with gas include and must be strictly followed:
- Do not bring a lit match or lighter to the cylinder to check for gas leaks.
- Strictly exclude the use of open flames to heat the gearbox or valve. For these purposes, only hot water can be used.
- If gas is detected in the room, do not turn on any electrical appliances, including lights, or turn them off. The temperature of a spark in a socket or switch can reach up to a thousand degrees.
- Do not try to repair shut-off valves and other structural elements of the cylinder yourself.
In addition, you must strictly follow the time frame for using the cylinders prescribed by the manufacturer. Vessels manufactured before December 2014 can be used for 40 years.
In the absence of information about the permitted period of use of gas cylinders produced after this date and without accompanying documentation, Rostechnadzor recommends taking 20 years as the shelf life of the cylinder.
A safe alternative to steel gas cylinders are more modern polymer-composite vessels - Eurocylinders. Their flasks are protected by a plastic casing and do not accumulate static electricity. The explosion safety of composite cylinders is ensured by equipping them with new generation safety devices - a fusible link and a check valve for releasing high pressure.
Acetylene toxicity
The toxicity of acetylene is due to the presence of harmful impurities in it. Acetylene causes drowsiness, and the harmful impurities contained in it, with prolonged inhalation, poison the body and cause nausea, dizziness, and sometimes severe general poisoning.
Therefore, the rooms where acetylene is produced and where work is carried out using it must have good ventilation.
Autoignition of acetylene
The auto-ignition temperature of acetylene ranges from 240-630°C depending on the pressure and the presence of various substances in it. It decreases with increasing pressure or with the presence of other substances in acetylene that increase the contact surface.
The dependence of the auto-ignition temperature on pressure is presented in the table below.
| Temperature, °C | Pressure, MPa |
| 630 | 0,2 |
| 530 | 0,3 |
| 475 | 0,4 |
| 350 | 2,2 |
In practice, depending on the pressure, it is permissible to heat acetylene to temperatures:
- up to 300°C - at an absolute pressure of 0.1 MPa (1 kgf/cm2);
- up to 150-180°C - at an absolute pressure of 0.25 MPa (2.5 kgf/cm2);
- up to 100°C - at higher pressures.
When burning acetylene mixed with oxygen in injection burners and cutters, it is necessary to take into account the possibility of flame penetration into the acetylene channel and its propagation in the direction opposite to the normal gas flow. This phenomenon is called flashback and can cause an explosion in the acetylene line or generator.
Why gas cylinders explode: main reasons and preventive measures
Agree, the news that a gas cylinder exploded somewhere, which we, unfortunately, sometimes hear on TV or from friends, makes us think about our own safety. And there is no place for complacency that this will not happen to us in this situation.
The consequences that such an explosion and the fire caused by it lead to can be the most tragic, and not only for property, but also for the health and life of people nearby. We will help you understand why gas cylinders can explode and how, without sacrificing the convenience of their use, you can protect yourself from big troubles.
To do this, we conducted a study of the features of existing types of household gas vessels, analyzed the reasons why explosions occurred in a number of real cases, and studied the competent opinions of experienced users about this. The proposed article can be presented as a compilation of sets of rules, presented in an understandable form, that allow, in practice, the correct and safe use of gas in cylinders.
Safety precautions when working with acetylene
Now, knowing how acetylene is dangerous, it became clear why special safety measures must be observed when working with it.
The basic safety requirements when working with this gas are set out in GOST 12.2.054, RD 34.03.204 and other regulatory documents, but here we will only briefly consider the most important points:
- It is prohibited to store oxygen cylinders in the same room as acetylene cylinders.
- On a special hand trolley, it is allowed to transport no more than one oxygen cylinder and one acetylene cylinder directly to the workplace.
- It is prohibited to completely use all the gas from the cylinder. The acetylene cylinder must have a residual pressure of at least:
Temperature, °C below 0 0-15 16-25 26-35 Minimum permissible residual pressure according to the pressure gauge, MPa (kgf/cm2) 0.049 0,049 (0,5) 0,098 (1,0) 0,196 (2,0) 0,294 (3,0) - In order to reduce losses of acetone, in which acetylene is dissolved, gas is taken from cylinders located only in a vertical position.
- Acetylene cylinders are allowed to be stored in special warehouses or in open areas under a canopy that protects them from precipitation and direct sunlight.
- For the manufacture of parts that come into direct contact with acetylene, the use of:
- copper and alloys in which the copper content is more than 65%;
- silver and its alloys;
- mercury;
- magnesium;
- zinc;
- and etc.
Rules for completing gas welding work
Extinguishing the burner should occur in the following sequence:
- The acetylene valve closes.
- The oxygen valve is closed.
- Close the valve on the oxygen cylinder.
- The gearbox is removed.
- The retort is unloaded on the generator (it is not allowed to be opened until the carbide has completely cooled down).
- The generator is cleaned and the housing is washed with water . For cleaning, use a scraper (brass or aluminum) or a hair brush.
- in which the generator was located is ventilated
Only after completing all the above steps can the work be considered completed.
Thus, gas welding is a particularly dangerous job. This welding method involves the use of explosive gases such as acetylene and oxygen. Compliance with safety regulations allows you to protect the welder’s work. Safety requirements apply to the room in which welding work will be performed, the welder’s clothing, the work process and the equipment used.
source