The classical theory of electrical conductivity of metals originated at the beginning of the twentieth century. Its founder was the German physicist Karl Rikke. He experimentally established that the passage of a charge through a metal does not involve the transfer of conductor atoms, unlike liquid electrolytes. However, this discovery did not explain what exactly is the carrier of electrical impulses in the metal structure.
The experiments of scientists Stewart and Tolman, conducted in 1916, allowed us to answer this question. They were able to establish that the smallest charged particles - electrons - are responsible for the transfer of electricity in metals. This discovery formed the basis of the classical electronic theory of electrical conductivity of metals. From this moment on, a new era of research into metal conductors began. Thanks to the results obtained, today we have the opportunity to use household appliances, production equipment, machines and many other devices.
What is metal?
Metal is a light body that can be forged. We find only six such bodies: gold, silver, copper, tin, iron and lead.
Interesting materials:
How much sleep should an orderly sleep? How long was Operation Barbarossa supposed to last? How long should red caviar drain? How many running tracks should there be at any international competition? How much should be in an essay? How many Dungans are there in Kyrgyzstan? How long does it take to get from Krasnodar to Simferopol? How long does it take to get from Boryspil airport to the railway station? How many elements were included in the first published version of Mendeleev's periodic table? How much Elo do you need for lvl 4?
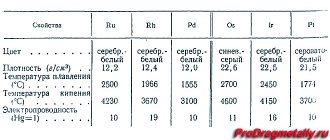
How does the electrical conductivity of different metals differ?
The electronic theory of electrical conductivity of metals was developed in the research of Paul Drude. He was able to discover such a property as resistance, which is observed when electric current passes through a conductor. In the future, this will make it possible to classify different substances according to their conductivity level. From the results obtained, it is easy to understand which metal is suitable for the manufacture of a particular cable. This is a very important point, since incorrectly selected material can cause a fire as a result of overheating from the passage of excess voltage current.
Silver metal has the highest electrical conductivity. At a temperature of +20 degrees Celsius, it is 63.3 * 104 centimeters-1. But making wiring from silver is very expensive, since it is a rather rare metal, which is used mainly for the production of jewelry and decorative items or bullion coins.
The metal with the highest electrical conductivity among all elements of the base group is copper. Its indicator is 57*104 centimeters-1 at a temperature of +20 degrees Celsius. Copper is one of the most common conductors used for household and industrial purposes. It withstands constant electrical loads well, is durable and reliable. The high melting point allows you to work for a long time in a heated state without problems.
In terms of abundance, only aluminum can compete with copper, which ranks fourth in electrical conductivity after gold. It is used in networks with low voltage, as it has almost half the melting point of copper and is not able to withstand extreme loads. The further distribution of places can be found by looking at the table of electrical conductivity of metals.
It is worth noting that any alloy has much lower conductivity than the pure substance. This is due to the merging of the structural network and, as a consequence, disruption of the normal functioning of electrons. For example, in the production of copper wire, a material with an impurity content of no more than 0.1% is used, and for some types of cable this indicator is even stricter - no more than 0.05%. All given indicators are the electrical conductivity of metals, which is calculated as the ratio between the current density and the magnitude of the electric field in the conductor.
Metals with high electrical conductivity
The electrical conductivity of alkali metals is at a high level, since their electrons are weakly attached to the nucleus and easily line up in the desired sequence. But this group is characterized by low melting points and enormous chemical activity, which in most cases does not allow their use for the manufacture of wires.
Metals with high electrical conductivity when opened are very dangerous for humans. Touching a bare wire will result in an electrical burn and a powerful discharge to all internal organs. This often results in instant death. Therefore, special insulating materials are used for the safety of people.
Depending on the application, they can be solid, liquid or gaseous. But all types are designed to do one thing—isolating electrical current within a circuit so that it cannot affect the outside world. The electrical conductivity of metals is used in almost all areas of modern human life, so ensuring safety is a top priority.
Formulas
There are three different formulas for electrical conductivity: σ (Greek sigma), k (kappa) and γ (gamma). In what follows we will use σ. The formula for electrical conductivity, also called electrical conductivity, is described by the formula:
σ = 1/ρ .
Here ρ is called resistivity. You can calculate the electrical resistance R of a conductor given its parameters as follows: R = (ρ * l) / S.
Thus, the resistance R is equal to the resistivity ρ, multiplied by the length of the conductor l, divided by the cross-sectional area S. If you now want to express this formula in terms of the electrical conductivity σ = 1/ρ, it is useful to know that the electrical conductivity G of the conductor is expressed as follows : G = 1/R .
If we substitute specific electrical conductivity σ and electrical conductivity G into the upper formula, we get the following: 1/G = (1/σ) * (l/S).
By further transformation we can obtain the expression: G = σ * S / l .
Electrical conductivity can also describe the important relationship between electric current density and electric field strength using the expression: J = σ * E.
Classical theory of electrical conductivity of metals
The basic principles of the theory of electrical conductivity of metals contain six points. First: a high level of electrical conductivity is associated with the presence of a large number of free electrons. Second: electric current arises through external influence on the metal, during which electrons move from random motion to ordered motion.
Third: the strength of the current passing through a metal conductor is calculated according to Ohm's law. Fourth: different numbers of elementary particles in the crystal lattice lead to unequal resistance of metals. Fifth: electric current in the circuit arises instantly after the start of exposure to electrons. Sixth: as the internal temperature of the metal increases, the level of its resistance also increases.
The nature of the electrical conductivity of metals is explained by the second point of the provisions. In a quiet state, all free electrons rotate chaotically around the nucleus. At this moment, the metal is not able to independently reproduce electrical charges. But as soon as you connect an external source of influence, the electrons instantly line up in a structured sequence and become carriers of electric current. With increasing temperature, the electrical conductivity of metals decreases.
This is due to the fact that the molecular bonds in the crystal lattice weaken, elementary particles begin to rotate in an even more chaotic order, so the formation of electrons in a chain becomes more complicated. Therefore, it is necessary to take measures to prevent overheating of the conductors, as this negatively affects their performance properties. The mechanism of electrical conductivity of metals cannot be changed due to the current laws of physics. But it is possible to neutralize negative external and internal influences that interfere with the normal course of the process.
What explains thermal conductivity?
So, the thermal conductivity of different substances is different. ... This is explained by the fact that thermal conductivity is the transfer of energy from one part of the body to another, which occurs during the interaction of molecules or other particles. In a space where there are no particles, thermal conduction cannot occur.
Interesting materials:
How many sites are there at Baikonur? How many planes pass through different triples of four points that do not belong to the same plane? How long does the history exam take? How long does it take to deep-fry nuggets? How many subscribers does Milana Nekrasova have in Like 2022? How many subscribers does Rahim have? How many trains are there in the metro? How much salt should I put in for salting lard? How much does a loader get paid at a fixed price? How much does a consultant earn at Sberbank in Moscow?
Physical meaning of conductivity
The use of metal conductors has a long history. Scientists and engineers working in fields of science and technology that use electricity have long decided on materials for wires, terminals, contacts, printed circuit boards, etc. A physical quantity called electrical conductivity helps determine the most electrically conductive metal in the world.
The concept of conductivity is the inverse of electrical resistance. The quantification of conductivity is related to the unit of resistance, which is measured in Ohms in the International System of Units (SI). The SI unit of electrical conductivity is siemens. The Russian designation for this unit is Cm, the international designation is S. An electrical conductivity of 1 Cm has a section of an electrical network with a resistance of 1 Ohm.
Conductivity
The measure of a substance's ability to conduct electric current is called electrical conductivity. The most electrically conductive metal has the highest similar indicator. This characteristic can be determined for any substance or environment instrumentally and has a numerical expression. The specific electrical conductivity of a cylindrical conductor of unit length and unit cross-sectional area is related to the resistivity of this conductor.
The system unit for conductivity is siemens per meter – S/m. To find out which metal is the most electrically conductive metal in the world, it is enough to compare their experimentally determined conductivities. You can determine the resistivity using a special device - a microohmmeter. These characteristics are inversely dependent.
Top best conductors - metals
4 metals that are of practical importance for their use as electrical conductors are distributed in the following order relative to the value of specific conductivity, measured in S/m:
- Silver - 62,500,000.
- Copper – 59,500,000.
- Gold – 45,500,000.
- Aluminum - 38,000,000.
It can be seen that the most electrically conductive metal is silver. But like gold, it is used to organize the electrical network only in special specific cases. The reason is high cost.
But copper and aluminum are the most common option for electrical appliances and cable products due to their low resistance to electric current and affordability. Other metals are rarely used as conductors.
Factors affecting the conductivity of metals
Even the most electrically conductive metal reduces its conductivity if it contains other additives and impurities. Alloys have a different crystal lattice structure than “pure” metals. It is characterized by a violation of symmetry, cracks and other defects. Conductivity also decreases with increasing ambient temperature.
The increased resistance inherent in alloys is used in heating elements. It is no coincidence that nichrome, fechral and other alloys are used to manufacture working elements of electric furnaces and heaters.
The most electrically conductive metal is precious silver, mostly used by jewelers, for minting coins, etc. But its special chemical and physical properties are also widely used in technology and instrument making. For example, in addition to being used in components and assemblies with reduced resistance, silver plating protects contact groups from oxidation. The unique properties of silver and alloys based on it often make its use justified, despite its high cost.