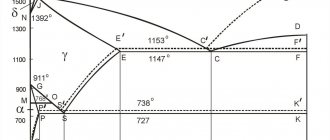
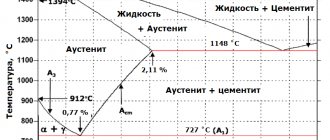
Iron-carbon diagram
is a graphical display of the structure of alloys consisting only of iron and carbon, depending on the initial average carbon concentration and the current temperature of the alloy.
The iron-carbon diagram allows you to understand the processes that occur during heat treatment of steel
.
Iron-carbon (iron-cementite) diagram. Simplified
Classification of iron-carbon alloys
Various combinations of these elements lead to a large number of alloys, which can be divided into three large groups:
- Technical hardware.
- Become.
- Cast iron.
Technical hardware
Technical iron includes materials containing less than 0.02% carbon. Steels include materials in which carbon is in the range from 0.02 to 2.14%. And the cast iron group includes materials in which the amount of carbon exceeds 2.14%.
Components in the iron-carbon system
Austenite
The atoms are placed in a face-centered cell. The hardness of austenite has a hardness of 200 ... 250 Brinell units. In addition, it has good ductility and is paramagnetic.
Iron
Iron is a material classified as a metal. Its natural color is silver-gray. In its pure form it is very plastic. Its specific gravity is 7.86 g/cc. cm. Melting point is 1539 °C. In practice, industrial iron is most often used, which contains the following impurities - manganese, silicon and many others. The mass fraction of impurities does not exceed 0.1%.
Iron
Iron has such a property as polyformism. That is, with the same chemical composition, this substance can have a different crystal lattice structure and, accordingly, different properties. Modifications of iron are called respectively - B, D, D. All these modifications exist under different conditions. For example, type B can only exist at a temperature of 911 °C. Type G can exist in the range from 911 to 1392 °C. Type D exists in the range from 1392 to 1539 °C.
Each type has its own crystal lattice shape, for example, type B has a cube-shaped lattice, type G lattice has a face-centered cubic shape. The D-type lattice has the shape of a volume-centered cube.
Another property is that at temperatures below 768, iron is ferrimagnetic, and as it increases, this property is lost.
The points of polymorphic and magnetic transformation are called critical. On the table they are designated as follows - A2, A3, A4. Digital indices indicate the type of transformation. To more fully distinguish the transformation of iron from one type to another, the indices c and r are added to the designation. The first one talks about heating, the second one talks about cooling.
Iron polymorphs
With high ductility parameters, iron does not have high hardness; on the Brinell scale it is equal to 80 units.
Iron has the ability to form solid solutions. They can be divided into two groups - substitution and implementation solutions. The former consist of iron and other metals, the latter of iron and carbon, hydrogen and nitrogen.
Carbon
Another component of the system is carbon. It is a non-metal and has three modifications in the form of diamond, graphite and coal. It melts at 3500 °C.
Allotropic modifications of carbon
In an iron alloy, this element is found in the form of a solid solution, it is called cementite or in the form of graphite. In this form it is present in gray cast iron. Graphite is neither ductile nor durable.
Cementite
The carbon share is 6.67%. It has high hardness - 800 HB, but at the same time it lacks ductility. Does not have polymorphic properties.
It has the following property - when forming a substitution solution, carbon can be replaced by atoms of other substances, for example, chromium or nickel. This solution is called a doped solution.
Cementite
It is not stable; under certain conditions it can decompose, and carbon is transformed into graphite. This property has found application in the formation of cast irons.
By the way, in a liquid state, iron can dissolve impurities in itself, while forming a homogeneous mass.
Ferrite
This is the name given to the solid solution in which carbon is introduced into iron.
It dissolves with a certain variability; at normal (room) temperature, the volume of carbon is within 0.006%; at 727 °C, the carbon concentration will be 0.02%. Upon reaching 1392 °C, ferrite is formed.
Ferrite
The carbon content will be 0.1%. Its atoms are located in defective lattice sites.
Ferrite is close in its parameters to iron.
Ferrite
It is a solid solution of carbon interstitial in α-ferrum. Small amounts of impurities may also be included. But ferrite has almost the same qualities as pure iron. If you examine the structure under a microscope, you can see light-colored polyhedral grains.
Happens:
- low temperature (at a temperature of 727° C, carbon solubility is 0.02%);
- high-temperature (at 1499 ° C, carbon solubility is 0.1%), or it is called δ-ferrum.
Ferrite properties:
- hardness - 80-120 HB;
- temporary resistance - 300 MPa;
- relative elongation - 50%;
- has good magnetic properties (up to a temperature of 768° C).
Austenite in steels
The presence of austenite in steel alloys gives them certain properties. Parts and assemblies made from such steels are intended to work in environments containing aggressive components, for example, in enterprises processing various acids.
Steels of this class are characterized by a high level of alloying; during crystallization, a face-centered lattice is formed. This structure is not subject to change even under the influence of deep cold.
Steels of this type can be divided into two types that differ from each other in composition. Firstly, they contain substances such as iron, nickel, chromium. In this case, the total number of additives cannot exceed 55%. The second group includes nickel and iron-nickel compositions. In nickel compositions, its content exceeds 55%. In iron-nickel compositions, the ratio of nickel to iron is 1:5, and the amount of nickel starts from 65%.
This amount of nickel provides increased ductility, and chromium, in turn, provides high corrosion resistance and heat resistance. The use of other alloying materials makes it possible to smelt alloys with unique performance properties. Metallurgists, when formulating alloys, are guided by the future purpose of the steels.
To produce alloy steels, ferritizers are used, which impart constancy to austenites; such substances include niobium, silicon and some others. In addition to them, carbon and manganese are used - they are called austenizers.
Cementite: forms of existence
This is the name given to the compound of carbon and iron. It is a component of cast iron and some steels. It contains 6.67% carbon.
Its crystal includes several octahedra; they are located with respect to each other at a certain angle. Inside each of them is a carbon atom. As a result of this construction, the following picture is obtained - one atom comes into contact with several atoms of iron, and iron, in turn, is connected with three atoms of this element.
Crystal lattice of cementite
This substance has all the properties that are inherent in metals - electrical conductivity, a peculiar shine, high thermal conductivity. That is, a mixture of iron and carbon behaves like a metal. This material has a certain fragility. Most of its properties are determined by the complex structure of the crystal lattice.
This material melts at 1600 degrees Celsius. But there are several opinions on this matter; some researchers believe that its melting point lies in the range from 1200 to 1450, others determine that the upper level is 1300 °C.
Primary cementite
Metallurgists distinguish three types of this substance - primary, secondary, tertiary.
Iron-cementite diagram
Primary, obtained from the liquid during hardening of alloys that contain 5.5% carbon. The primary one has the shape of large plates.
Secondary
This element is obtained from austenite when the latter is cooled. On the diagram, this process can be seen in the Fe – C diagram. Cementite is presented in the form of a grid placed along the grain boundaries.
Tertiary
This type is derived from ferrite. It is shaped like needles.
There are other forms of cementite in metallurgy, for example, Stead's cementite, etc.
Other structural components in the iron-carbon system
Perlite
Perlite is a mechanical mixture that consists of ferrite and cementite. Ledeburite is a variable solution.
Perlite
At temperatures from 1130 to 723 ° C, its composition includes austenite and cementite. At lower temperatures it consists of austenite replacing ferrite.
Main lines of the iron-cementite diagram
ABCD
line is the liquidus line. It says that any alloy at temperatures above this line is completely in a liquid state. When the alloy crosses this line during the heating process, the melting process ends here, and when the alloy crosses this line during the cooling process, the crystallization process will begin here.
AHJECF Line
– this is the solidus line. It says that any alloy at temperatures below this line is completely solid. When the alloy crosses this line during the cooling process, the crystallization process ends here, and when the alloy crosses this line during the heating process, the melting process will begin here.
HJB line
is the boundary of the peritectic reaction. On this line, at a constant temperature of 1499 °C, a peritectic transformation occurs, which consists in the fact that the liquid phase (L) reacts with previously formed ferrite crystals (δ), resulting in the formation of austenite (A): .
ECF line
is the boundary of the eutectic reaction. In this section, at a constant temperature of 1147 ° C, a eutectic transformation occurs, which consists in the fact that a liquid containing 4.3% carbon turns into a eutectic mixture of austenite and cementite: .
The eutectic of the iron-cementite system is called ledeburite (L), named after the German scientist A. Ledebur, and contains 4.3% carbon.
PSK line
is the boundary of the eutectoid reaction. Along the PSK line at a constant temperature of 727 °C there is a eutectoid transformation, which consists in the fact that austenite containing 0.8% carbon is transformed into a eutectoid mixture of ferrite and cementite: .
According to the mechanism, this transformation is similar to the eutectic one, but occurs in the solid state.
The eutectoid of the iron-cementite system is called pearlite (P) and contains 0.8% carbon. It got its name because a pearlescent sheen is observed on polished and etched sections.
Perlite can exist in granular and lamellar form, depending on the conditions of formation.
AHN
line is
the limit of carbon solubility in δ-iron. The solid solution of carbon in δ-iron is called high-temperature ferrite. On domestic diagrams it is most often denoted by the Greek letter δ.
NJESG Line
– this is the limit of carbon solubility in γ-iron. The solid solution of carbon in γ-iron is called austenite. On domestic diagrams it is usually denoted by the capital letter A.
QPG line
– this is the limit of the limiting solubility of carbon in α-iron. The solid solution of carbon in α-iron is called ferrite. On domestic diagrams it is most often denoted by the capital letter F.
Line ES
– boundary of austenite supersaturation with carbon. When the alloy crosses this line during the cooling process, secondary cementite is released on it, and during the heating process, secondary cementite disappears.
PQ line
– this is the boundary of ferrite supersaturation with carbon. When the alloy crosses this line during the cooling process, tertiary cementite will be released from the ferrite, and during the heating process, tertiary cementite will disappear.
Nodal critical points of the iron-carbon system state diagram
On the iron-carbon diagram there are a number of points called critical points. Each point carries information about temperature, carbon content and a description of what exactly is happening in that place.
There are 14 of these critical points in total.
For example, A, says that at a temperature of 1539 ° C and at zero carbon content, pure iron melts. D indicates that at a temperature of 1260 Fe3c can melt.
The points are located at the intersection of lines placed on the diagram.
Cast iron
Alloys on the iron-carbon diagram that contain more than 2.14% carbon are called cast irons. They are highly fragile. The cross section of such cast iron has a light tone, and therefore it is called white cast iron.
On the diagram this is point C, called the eutectic, with a corresponding carbon content of 4.3%. Crystallization produces a mixture of austenite and cementite, collectively called ledeburite. The phase composition is constant.
When the carbon concentration is less than 4.3% (hypoeutectic cast iron), austenite is released from the solution during crystallization. Next, C2 is isolated from it. And at 727° C, austenite turns into pearlite. The structural state of such cast iron is as follows: large areas of dark-colored pearlite.
In hypereutectic white cast iron (carbon more than 4.3%), upon cooling, structuring occurs with the formation of C1 crystals. Further transformations are carried out in the solid state. The structure is ledeburite, which is the background for fields of dark-toned pearlite. And large layers are Ts1.