02/18/2022 Author: VT-METALL
From this material you will learn
:
- History of the discovery of iron-carbon alloys
- Structural components of iron-carbon alloys
- Iron-carbon alloy diagram
- How to Read an Iron-Carbon Alloy Diagram
- Application of iron-carbon alloy steel
- Varieties of cast iron made from an alloy of iron and carbon
- Polymorphic transformations in iron-carbon alloys
The discovery of an alloy of iron and carbon was one of the most important events in the history of the development of metallurgy. It was these two elements that gave the world the most popular grades of steel and cast iron. These are the alloys from which most industrial equipment, metal structures, tools, and household products are produced.
Depending on the percentage of carbon in the iron, as well as the method of casting, these alloys acquire different properties: resistance to corrosion, extraordinary strength, elasticity, etc. You will learn about which alloys of iron and carbon are used today and how they are obtained from our material.
History of discovery
For the first time, the great metallurgist and inventor Dmitry Konstantinovich Chernov (1868) pointed out that there are certain (special) points in alloys (steels and cast irons).
It was he who made an important discovery about polymorphic transformations and is one of the creators of the iron-carbon phase diagram. According to Chernov, the position of these points on the diagram is directly dependent on the percentage of carbon content. And what is most interesting is that it is from the moment of this discovery that the science of metallography begins its life.
The diagram of iron-carbon alloys is the result of the painstaking work of scientists from several countries around the world. All letter designations of the main points and phases in the diagram are international.
Polymorphic transformations
More details about each phase are given below in the article. In short, the main transformations occur at special temperatures.
The state of iron is designated as α-ferrum (at a temperature less than 911 ° C). The crystal lattice is a volumetric face-centered cube. Or OCC. The distance between the atoms of such a lattice is quite high.
Iron acquires the gamma modification, that is, it is designated as γ-ferrum (911-1392° C). The crystal lattice is a face-centered cube (FCC). In this lattice the distance between atoms is lower than in bcc.
When α-ferrum transforms into γ-ferrum, the volume of the substance becomes smaller. The reason for this is the crystal lattice - its appearance. Because the fcc lattice has a more ordered state of atoms than the bcc lattice.
If the transition is carried out in the opposite direction - from γ-ferrum to α-ferrum, then the volume of the alloy increases.
When the temperature reaches 1392° C (but less than the melting point of iron 1539° C), then α-ferrum turns into δ-ferrum, but this is not its new form, but only a variety. In addition, δ-ferrum is an unstable structure.
Structures in an iron-carbon diagram
Let us recall the 2 crystalline forms of iron:
- α-iron. Has a body-centered cubic (bcc) lattice;
- γ-iron It has a face-centered cubic (fcc) lattice.
Crystal lattice of iron The polymorphic transformation of one form into another during heat treatment of steels occurs when the alloys pass through the GSK line.
Let us distinguish 4 phases in the iron-carbon system:
- Liquid phase. Carbon concentration is not limited;
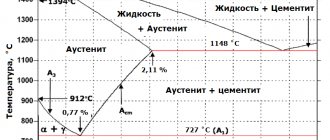
- Ferrite is a solid solution of carbon in α-iron. The maximum carbon concentration is only 0.025% (point P). At room temperature – not higher than 0.006%. Ferrite is soft and ductile.
- Austenite is a solid solution of carbon in γ-iron. The maximum carbon concentration is 2.14% (point E). Austenite has low hardness, is plastic, and is not magnetic.
- Cementite is a chemical compound of iron and carbon (iron carbide, Fe3C). The carbon concentration is, accordingly, constant – 6.67% carbon. Cementite is very hard, brittle, and non-plastic.
Depending on the conditions of education, there are:
- primary cementite (formed from liquid);
- secondary cementite (precipitated from austenite around its grains);
- tertiary cementite (precipitated from ferrite along the boundaries of its grains);
- eutectoid cementite (is a component of pearlite);
- eutectic cementite (is a component of ledeburite).
It is also necessary to distinguish 2 structural components of iron-carbon alloys:
- Pearlite (eutectoid) is a mechanical mixture of 2 phases - plates/grains of ferrite and cementite. Pearlite is formed as a result of the pearlitic transformation of austenite (“free” or included in ledeburite) with a carbon concentration of 0.8% when passing below the PSK line:
A0.8→F0.025 + C6.67
Perlite structure. F - ferrite, C - cementite
In this case, iron passes from the γ-form to the α-form. Mechanical properties strongly depend on the size (dispersity) of the particles that make up a given pearlite.
- Ledeburite (eutectic) is a mechanical mixture of 2 phases – plates/grains of austenite and cementite. Ledeburite forms from a liquid phase with a carbon concentration of 4.3% when passing below the ECF line:
Zh4.3→A2.14 + C6.67
Structure of ledeburite. C - cementite, A - austenite.
Repeating, we recall that when alloys pass below the PSK line (727°C), austenite, which is part of ledeburite, undergoes a pearlite transformation, separating into ferrite and cementite. Ledeburite is hard and brittle.
At room temperature, iron-carbon alloys can have different structures, and therefore properties, although they always consist of only 2 phases: ferrite and cementite.
Cementite: forms of existence
This is the name given to the compound of carbon and iron. It is a component of cast iron and some steels. It contains 6.67% carbon.
Its crystal includes several octahedra; they are located with respect to each other at a certain angle. Inside each of them is a carbon atom. As a result of this construction, the following picture is obtained - one atom comes into contact with several atoms of iron, and iron, in turn, is connected with three atoms of this element.
Crystal lattice of cementite
This substance has all the properties that are inherent in metals - electrical conductivity, a peculiar shine, high thermal conductivity. That is, a mixture of iron and carbon behaves like a metal. This material has a certain fragility. Most of its properties are determined by the complex structure of the crystal lattice.
This material melts at 1600 degrees Celsius. But there are several opinions on this matter; some researchers believe that its melting point lies in the range from 1200 to 1450, others determine that the upper level is 1300 °C.
Primary cementite
Metallurgists distinguish three types of this substance - primary, secondary, tertiary.
Iron-cementite diagram
Primary, obtained from the liquid during hardening of alloys that contain 5.5% carbon. The primary one has the shape of large plates.
Secondary
This element is obtained from austenite when the latter is cooled. On the diagram, this process can be seen in the Fe – C diagram. Cementite is presented in the form of a grid placed along the grain boundaries.
Tertiary
This type is derived from ferrite. It is shaped like needles.
There are other forms of cementite in metallurgy, for example, Stead's cementite, etc.
Other structural components in the iron-carbon system
Perlite
Perlite is a mechanical mixture that consists of ferrite and cementite. Ledeburite is a variable solution.
Perlite
At temperatures from 1130 to 723 ° C, its composition includes austenite and cementite. At lower temperatures it consists of austenite replacing ferrite.
Some elements of the iron-carbon diagram
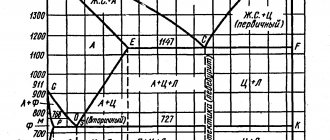
Let's highlight several boundaries on the iron-carbon diagram:
- ACD line. Liquidus line. When the alloys below it are cooled, their crystallization begins;
- AECF line. Solidus line. When cooling alloys below it, the entire alloy turns into a solid state;
- ECF line. Sometimes called ledeburite transformation line. When alloys with a carbon content above 2.14% are cooled, the liquid phase below it turns into ledeburite;
- PSK line. Pearlite transformation line. When alloys below it are cooled, austenite transforms into pearlite.
Let's note a few important points on the diagram:
- point E. The point of maximum saturation of austenite with carbon is 2.14%, at a temperature of 1147°C;
- point P. The point of maximum saturation of ferrite with carbon is 0.025%, at a temperature of 727 ° C;
- point S. Point “0.8% С-727°С” of transformation of austenite with a carbon concentration of 0.8% into pearlite (eutectoid) of the same average concentration;
- point C. Point “2.14% С-1147°С” for the transformation of a liquid with a carbon concentration of 2.14% into ledeburite (eutectic) of the same average concentration.
Often the temperature values at which structural changes occur in a particular alloy are denoted by the letters A:
- A1 – PSK line;
- A2 – line MO – Curie point, at which a change in the magnetic properties of alloys occurs;
- A3 – temperatures corresponding to the GS line;
- Acm – temperatures corresponding to the SE line.
Since the temperatures of phase transitions during heating and cooling are slightly different, additional letter designations are often introduced:
- с – for temperatures of phase transitions during heating;
- r – during cooling,
for example Ac1 or Ar1.
Meaning of chart lines
Boundaries that intersect each other mark specific areas on the diagram. Within each zone there can be a single phase or two phases. A phase transition occurs at the boundary. These regions are phase fields, they indicate the phases present for a particular alloy composition and temperature.
There are several characteristic lines on the diagram, designated A1, A2, A3, A4 and ACM. When the temperature of the metal increases or decreases, a phase transition occurs at these boundaries. Typically, when an alloy is heated, its temperature rises, but along these lines, heating causes the structure to rearrange itself into a different phase, and thus the temperature stops increasing until the phase completely changes. This process is called thermal shutdown.
The alloy steel elements—nickel, manganese, chromium, and molybdenum—affect the position of these boundaries on the phase diagram. Borders can move in any direction depending on the element used. For example, in the phase diagram of iron and carbon, adding nickel lowers the A3 limit, and adding chromium increases it.
Application of phase diagram of iron-carbon alloys
The state diagram of alloys of the iron-cementite system is used to determine the heat treatment mode of the alloy, the heating temperature of the metal for forging and the temperature limit of forging, as well as the melting temperature, which is necessary to determine the mode of pouring the liquid alloy into molds.
Heat treatment is performed by heating metal alloys to specific temperatures, holding them at these temperatures, and then rapidly or slowly cooling them to change the properties of the alloy in the desired direction.
The heat treatment of iron-carbon alloys has a number of variations, based on the fact that the austenite structure, which is unstable at low temperatures, depending on the cooling rate of the alloy, transforms into structures with different properties. The decomposition products of austenite are martensite, troostite, sorbitol and pearlite.
Martensite is a product of hardening of austenite and its transformation into ferrite without the release of carbon from solution. Therefore, martensite is α-iron highly supersaturated with carbon with a fermented crystal lattice. This determines its high hardness (HB 600-700) and strength, increased strength and the presence of internal stresses. This structure is formed at high cooling and hardening rates (180 ÷ sec for carbon steel). Martensite by its nature is unstable and, when heated to temperatures above 70°, tends to transform into other structures.
Troostite is a mechanical mixture of ferrite cementite with a very high degree of dispersion. The hardness of troostite HB is 350÷500. This structure is formed at a quenching rate of carbon steel of about 80°/sec. Acicular troostite is sometimes called bainite.
Sorbitol is a coarser mechanical mixture of ferrite and cementite grains, but quite dispersed. It is difficult to distinguish under a conventional microscope. The hardness of sorbitol is 250÷350. This structure is formed at carbon steel quenching rates of less than 50°/sec. Compared to troostite, copbit has a higher viscosity, and compared to perlite, greater hardness.
Perlite is a more or less rough mechanical mixture of ferrite and cementite. Pearlite is formed at low cooling rates of steel heated to the austenitic state.
Troostite, sorbitol and pearlite can be prepared by tempering martensite at increasing tempering temperatures. In this case, they have excellent, often higher mechanical properties than when austenite is cooled at different rates.
Thus, by changing the heat treatment regime, it is possible to obtain different physical and mechanical properties and structures of steel. Heat treatment operations include annealing, normalizing, hardening and tempering.
Annealing - phase recrystallization - consists of heating hypoeutectoid steel above the A3 line, and hypereutectoid steel above the Ast line, followed by slow cooling along with the furnace. If you heat steel above A1, but below A3 (or Ast), then complete recrystallization will not occur. This heat treatment is called incomplete annealing. During annealing, the state of the steel approaches equilibrium. Therefore, the structure of annealed steel consists of either ferrite and pearlite (hypoeutectoid steels) or pearlite and secondary cementite (hypoeutectoid steels).
Temperature limits of complete annealing, incomplete annealing, high tempering and normalization, plotted on the section of the iron-cementite phase diagram
Annealing reduces the hardness and increases the toughness of steel, improves its machinability, relieves internal stresses, and also eliminates structural heterogeneity and stabilizes physical properties.
Normalization differs from annealing by an increased cooling rate (in still or moving air). Normalization is used to refine metal grains and increase its strength.
Hardening is the heating of steel above the critical point A3 (Fig. 9), followed by rapid cooling in water, oil or other cooling media. Typically, the purpose of hardening is to obtain a martensitic structure, which is then tempered. Incomplete hardening occurs if hypoeutectoid steel has been heated to a temperature above point Ai but below point A3. Ferrite, contained in such steel along with austenite, naturally does not accept hardening. Hypereutectoid steels are hardened at temperatures above A1, but below Acm, since it is inappropriate to dissolve solid inclusions of secondary cementite during heating.
Temperature limits for forging and hot stamping, plotted on the section of the iron-cementite phase diagram.
When tempering, the steel is heated to a temperature below A1, maintained at this temperature and slowly cooled along with the furnace. Low tempering (175-250°) serves to increase the toughness of steel while maintaining high tensile strength and hardness, reducing internal stresses and obtaining more stable structures. High tempering (up to 700°) is used to increase the ductility and machinability of steel and reduce strength and hardness.
Forging, hot stamping and rolling of steel are carried out at relatively high temperatures. The steel is heated to a temperature 100-150° below the solidus line.
The end of the steel pressure treatment should occur at temperatures close to A3 for hypoeutectoid steel; the process at too low temperatures leads to the structure of the steel becoming rougher and its ductility decreases; the process at too high temperatures leads to an increase in the grain of the steel (overheating) and an increase in its fragility. Overheating can be corrected by heat treatment (annealing, normalization).
When steel is heated to a temperature close to the solidus line AE, oxidation of the metal occurs along the grain boundaries, as a result of which the connection between the latter is disrupted and the mechanical strength drops catastrophically. This phenomenon is called burnout, and it cannot be corrected by any subsequent heat treatment.
Types of steels with additional processing
Heat-resistant steels are intended for use in chemical and gaseous environments at high temperatures and in lightly loaded conditions. They are marked similarly to stainless steel, but unlike stainless steel, they undergo special hardening to obtain the desired characteristics. Heat-resistant steel is used in work with constant high temperatures. It can withstand operation at 1700 degrees Celsius for a long time. The types of this steel coincide with the previous two types, and are marked using alphanumeric designations that demonstrate the level of various impurities in the alloy and very permissible operating temperatures. All of the listed varieties are also used for marking sheet steel.
Interesting read: Electric steelmaking
Types of steel that have undergone additional processing after smelting also have their own markings. Zinc-plated steel is coated with a layer of zinc, which protects against corrosion. This type has been intensively used in the automotive industry for many years. Its widest distribution is hampered by the relative cost of production.
Grades of zinc-coated steel are designated by a letter abbreviation, which characterizes the purpose of the material, its ability to draw and the uniformity of the zinc-coated coating. These characteristics determine the possibility of introducing galvanization in a particular production. There are not so few of them, so you can simply learn all the abbreviations and navigate without any problems as you need to purchase.
The channel is a long product with a U-shaped section. It is used for the production of various prefabricated and welded structures at construction sites. The marking of the channel corresponds to the grade of steel from which it was made. It is designed to work under the influence of the highest overload, therefore the hardest grades are used for production. Other materials made on a metal base also often retain its brand or do not have it at all. Knowing the characteristics of steel grades will help you make the right choice when purchasing products for your intended purpose.
Classification of iron-carbon alloys
Various combinations of these elements lead to a large number of alloys, which can be divided into three large groups:
- Technical hardware.
- Become.
- Cast iron.
Technical hardware
Technical iron includes materials containing less than 0.02% carbon. Steels include materials in which carbon is in the range from 0.02 to 2.14%. And the cast iron group includes materials in which the amount of carbon exceeds 2.14%.
Use of steel and cast iron in everyday life
Steel, as a material, has entered our lives so closely that we don’t even suspect it. Man has been using metal products in everyday life for many centuries. Once a day, any of us comes across different types of metals and alloys, almost all of which are found at home or in the local area.
Phases of the iron-carbon diagram
In the iron-carbon system there are the following phases: liquid phase, ferrite, austenite, cementite, graphite.
Liquid phase. In the liquid state, iron dissolves carbon well in any proportions [source not specified 1441 days] to form a homogeneous liquid phase.
Ferrite is a solid solution of carbon interpolation in α-iron with a body-centered cubic lattice.
Ferrite has a variable, temperature-dependent limiting solubility of carbon: a minimum of 0.006% at room temperature (point Q), a maximum of 0.02% at a temperature of 700 °C (point P). Carbon atoms are located in the center of the face or (which is crystal geometrically equivalent) in the middle of the edges of the cube, as well as in lattice defects.
Above 1392°C there is high temperature ferrite with a carbon solubility limit of about 0.1% at about 1500°C (point H).
The properties of ferrite are close to those of pure iron. It is soft (Brinell hardness - 130 HB) and ductile, ferromagnetic (in the absence of carbon) up to the Curie point - 770 ° C.
Austenite (γ) is a solid solution of interstitial carbon in γ-iron with a face-centered cubic lattice.
Carbon atoms occupy a place in the center of a face-centered cubic cell. The limiting solubility of carbon in austenite is 2.14% at a temperature of 1147 °C (point E). Austenite has a hardness of 200-250 HB, is plastic, and paramagnetic. When other elements are dissolved in austenite or ferrite, the properties and temperature limits of their existence change [3].
Cementite (Fe3C) is a chemical compound of iron and carbon (iron carbide), with a complex rhombic lattice, containing 6.67% carbon. It is hard (over 1000 HB) and very brittle. Cementite is a metastable phase and, upon prolonged heating, spontaneously decomposes with the release of graphite.
In iron-carbon alloys, cementite as a phase can precipitate under various conditions:
- primary cementite (is released from the liquid),
- secondary cementite (separated from austenite),
- tertiary cementite (from ferrite),
- eutectic cementite and
- eutectoid cementite.
Primary cementite is released from the liquid phase in the form of large lamellar crystals. Secondary cementite is separated from austenite and is located in the form of a network around austenite grains (after the eutectoid transformation they will become pearlite grains). Tertiary cementite is released from ferrite and in the form of small inclusions is located at the boundaries of ferrite grains [4].
Eutectic cementite is observed only in white cast iron. Eutectoid cementite has a lamellar shape and is a component of pearlite. Cementite can be released in the form of small spheres during special spheroidizing annealing or hardening with high tempering. The mechanical properties of alloys are influenced by the shape, size, number and location of cementite inclusions, which allows in practice for each specific application of the alloy to achieve the optimal combination of hardness, strength, resistance to brittle fracture, etc. [5]
Graphite is a phase consisting only of carbon with a layered hexagonal lattice. The density of graphite (2.3 g/cm3) is much lower than the density of all other phases (about 7.5-7.8 g/cm3) and this complicates and slows down its formation, which leads to the release of cementite during faster cooling. The formation of graphite reduces shrinkage during crystallization, graphite acts as a lubricant during friction, reducing wear, and helps dissipate vibration energy.
Graphite comes in the form of large crab-shaped (curved plate-like) inclusions (regular gray cast iron) or spheres (ductile cast iron).
Graphite is necessarily present in gray cast iron and its variety - high-strength cast iron. Graphite is also present in some grades of steel - in the so-called graphitized steels.
Use of steel in everyday life
Different steel is used in the production of dishes and kitchen utensils: dark rolled metal, enameled steel, stainless steel, zinc coated sheet steel. Household kitchen utensils include all types of devices that are used at different stages of food preparation: a kitchen meat grinder, a device used to chop vegetables, household kitchen scales, a hanging or tabletop drying rack for plates and forks, spoons, etc.
Cooking utensils
Kitchen equipment: ovens, microwaves, dishwashers, steamers, etc. - all have a body almost always made of cold-rolled sheet.
In the kitchen, utility knives are used for various purposes: a bread knife, a meat knife, a knife for cutting cheese and butter, a knife for peeling fruits and vegetables, and various models of folding knives.
Kitchen tableware includes various types of spoons and forks. Spoons are divided by size and purpose: table spoons, dessert spoons, tea spoons and coffee spoons. Forks are divided into two types: table forks and buffet forks. Kitchen table sets, which include knives, forks and spoons, are also used in everyday life.
There are a number of types of household scissors: kitchen scissors, tailor scissors, manicure scissors, scissors used in hairdressing salons, etc.
Metal products and tools that are used for repair and construction
- various fastening products: nuts, nails, bolts, screws, etc.;
- fittings for windows and doors: handles, hinges, locks, latches, etc.;
- iron tools that are used for processing wood: saws, axes, etc.;
- build tools for processing iron parts: chisels, clamping vices, sledgehammers, hammers, anvils, hacksaws, ratfils, drilling tools and thread cutting devices;
- tools needed for installation: pliers, screwdrivers, wire cutters, pliers;
- construction equipment that contains steel elements: drill, hammer drill, screwdriver, etc.;
- gardening tools: shovels, rakes, pitchforks, scythes, hoes, sickles, etc.
Garden metal tools
Heating and lighting devices
- gas cookers;
- water heaters for home;
- electronic boilers for premises;
- heating boilers that operate on hard and watery fuels;
- kerosene lamps and street lamps.
Chimneys
In the installation of chimneys, a profile pipe is used, including one made of special heat-resistant steel, which is resistant to high-temperature corrosion.
Furniture
Various iron fittings are used in furniture systems. The main element of the fittings is a small profile iron pipe, an iron angle and an iron strip. Iron decor is often used in furniture.
Reading an Iron-Carbon Diagram
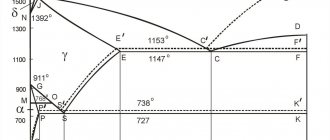
We can see the composition of an alloy with a given initial carbon content at a given temperature by moving along a vertical line corresponding to the carbon content in the alloy.
Consider, for example, the AEC region. It is adjacent to areas of austenite AESG and the liquid phase. The alloys in it consist of a liquid phase and the resulting solid austenite. How to determine the carbon concentration in different phases for a given alloy? Let us consider, as an example, an alloy with an initial carbon concentration of 2.5% at a temperature of 1250°C.
Let’s draw a horizontal straight line from this point on the graph “2.5% C – 1250°C”. The intersection of this straight line with the AE line bordering the austenite region will show the carbon concentration in austenite at a given temperature (~1.5%).
The intersection of the same horizontal line with line AC, bordering the liquid phase region, will show the concentration of carbon in the liquid phase at a given temperature (~3.5%).
This is how we can determine the carbon concentration in the phases of any alloy at a given temperature:
- in the liquid phase and austenite in the AEC region;
- in the liquid phase in the CDF region (carbon concentration in cementite, of course, is constant - 6.67%);
- in austenite in the SEFK region;
- in ferrite in the QPKL region;
- in ferrite and austenite in the GPS region.
As we can see, at a carbon concentration above 2.14%, the saturation of the cooled melt with carbon always tends to 4.3% (along the AC and DC lines) as it approaches a temperature of 1147°C (ECF level). Next, the liquid transforms into ledeburite (eutectic). Naturally, with the same average carbon content.
As the temperature approaches 727°C (PSK level), the carbon concentration in austenite (“free” and/or included in ledeburite) tends to 0.8% (according to the GS and ES lines). Next, the transformation of austenite into pearlite (eutectoid) occurs. Perlite, of course, has an average carbon content of 0.8%.
Stainless steel grades
In total, there are 5 types of stainless steel. Austenitic steels are characterized by the highest content of chromium (20-25%) and nickel (18%). They perfectly resist corrosion even at elevated temperatures. Ferritic steels differ in the presence of magnetic parameters and low carbon content. Chromium occupies about 17% of the alloy, and nickel is absent or its fraction is less than 0.1%.
Duplex steels are a mixed type, possessing the qualities of two previous grades. Martensitic steels contain only 12% chromium and a fairly high level of carbon. This species is used for the production of cutlery and surgical instruments. Hardened steels contain additional alloys (titanium, niobium and others) that increase the strength of the alloy. They are used in conditions where the exploited material will be under the influence of physical overload, for example, in the elements of mine reinforcement.
Properties of commercially pure iron
Magnetic properties of iron at different temperatures:
- less than 768° C – ferromagnetic;
- more than 768° C – paramagnetic.
And the temperature point of 768° C is called the magnetic transformation point, or the Curie point.
Properties of technically pure iron:
- hardness – 80 HB;
- temporary resistance - 250 MPa;
- yield strength – 120 MPa;
- relative elongation 50%;
- relative narrowing – 80%;
- high modulus of elasticity.
Chemical properties
As a chemical compound, cementite has its own physical, chemical and mechanical characteristics. It has a gray crystalline appearance at fracture and is relatively hard with high thermal stability. The main chemical properties of cementite are expressed in the following indicators:
- chemical formula Fe3C;
- decomposition of the structure occurs at temperatures above 1650°C;
- exposed to various acids (especially highly concentrated ones);
- quickly reacts with oxygen.
Based on the existing chemical properties, physical and mechanical properties are formed. The main physical properties include:
- melting point is 1700 °C;
- molecular weight is 179.55 amu;
- The density of cementite is 7.7 g/cm3 at a temperature of 20 °C.
The main mechanical properties include:
- hardness;
- resistance to impact (fragility);
- fracture resistance;
- plastic.
The hardness of this compound reaches high values and is equal to HB 8000 MPa or HRC 70. However, it has sufficient brittleness and low ductility.
Possessing the listed properties, cementite is actively used in the production of cast parts for various purposes. The formation of various types of cementite and its compounds with other forms leads to a change in the characteristics of the resulting steel or cast iron, therefore, to an improvement or decrease in individual consumer properties.
For example, to obtain white cast iron and give it high strength and ductility, they try to convert cementite into graphite. This is achieved during the annealing operation. As the temperature increases, it breaks down into two components: ferrite and graphite.
Depending on the required properties, they try to maintain the required amount of cementite in cast iron. This is especially true for the so-called free fraction of this compound. To reduce its concentration, various methods of chemical and thermal treatment are used. To solve this problem, use a solution of nitric acid in pure alcohol. Structurally free cementite precipitates as a result of boiling a cast iron pig in this solution. In addition, three types of processing are used: annealing, normalizing and hardening.
Technical iron contains tertiary cementite in combination with ferrite. It appears along the ferrite boundary at carbon contents from 0.01% to 0.025%. To improve the quality of steel, they try to reduce the content of free cementite. Its concentration is especially observed in soft steel grades. The content of this mixture and perlite per unit volume has a great influence on the quality of stamping. Excessive presence of tertiary cementite, especially in the form of a long chain or network, leads to the formation of breaks during stamping. Therefore, to obtain good forging steel, they try to reduce the amount of tertiary cementite. The structure of such formations should not exceed the second point on the established scale. The resulting hardness should not exceed HB 50 units.
Components in the iron-carbon system
Austenite
The atoms are placed in a face-centered cell. The hardness of austenite has a hardness of 200 ... 250 Brinell units. In addition, it has good ductility and is paramagnetic.
Iron
Iron is a material classified as a metal. Its natural color is silver-gray. In its pure form it is very plastic. Its specific gravity is 7.86 g/cc. cm. Melting point is 1539 °C. In practice, industrial iron is most often used, which contains the following impurities - manganese, silicon and many others. The mass fraction of impurities does not exceed 0.1%.
Iron
Iron has such a property as polyformism. That is, with the same chemical composition, this substance can have a different crystal lattice structure and, accordingly, different properties. Modifications of iron are called respectively - B, D, D. All these modifications exist under different conditions. For example, type B can only exist at a temperature of 911 °C. Type G can exist in the range from 911 to 1392 °C. Type D exists in the range from 1392 to 1539 °C.
Each type has its own crystal lattice shape, for example, type B has a cube-shaped lattice, type G lattice has a face-centered cubic shape. The D-type lattice has the shape of a volume-centered cube.
Another property is that at temperatures below 768, iron is ferrimagnetic, and as it increases, this property is lost.
The points of polymorphic and magnetic transformation are called critical. On the table they are designated as follows - A2, A3, A4. Digital indices indicate the type of transformation. To more fully distinguish the transformation of iron from one type to another, the indices c and r are added to the designation. The first one talks about heating, the second one talks about cooling.
Iron polymorphs
With high ductility parameters, iron does not have high hardness; on the Brinell scale it is equal to 80 units.
Iron has the ability to form solid solutions. They can be divided into two groups - substitution and implementation solutions. The former consist of iron and other metals, the latter of iron and carbon, hydrogen and nitrogen.
Carbon
Another component of the system is carbon. It is a non-metal and has three modifications in the form of diamond, graphite and coal. It melts at 3500 °C.
Allotropic modifications of carbon
In an iron alloy, this element is found in the form of a solid solution, it is called cementite or in the form of graphite. In this form it is present in gray cast iron. Graphite is neither ductile nor durable.
Cementite
Cementite (Fe3C) is a chemical compound of iron and carbon (iron carbide), contains 6.67% carbon. More precise studies have shown that cementite can have a variable carbon concentration. However, in the future, when analyzing the phase diagram, we will make the assumption that Fe3C has a constant composition. The crystal lattice of cementite is rhombic, specific gravity 7.82 g/cm3 (very close to the specific gravity of iron). At high temperatures, cementite dissociates, so its melting point is unclear and is approximately 1260° C. It does not experience allotropic transformations. The crystal lattice of cementite consists of a series of octahedra, the axes of which are inclined to each other. At low temperatures, cementite is weakly ferromagnetic, losing its magnetic properties at a temperature of about 210° C. Cementite has high hardness (more than 800 HB, easily scratches glass), but extremely low, almost zero, ductility.
Cementite is capable of forming substitutional solid solutions. Carbon atoms can be replaced by non-metal atoms: for example, nitrogen; iron atoms - metals: manganese, chromium, tungsten, etc. Such a solid solution based on a cementite lattice is called alloyed cementite.
If graphite is a stable phase, then cementite is a metastable phase. Cementite is an unstable compound and, under certain conditions, decomposes to form free carbon in the form of graphite. This process is of great practical importance in the formation of the structure of cast iron.
Primary cementite
Metallurgists distinguish three types of this substance - primary, secondary, tertiary.
Varieties of cast iron made from an alloy of iron and carbon
There are two main types of cast iron – foundry and ultimate. The first type is usually used in production and industrial applications. The second is used in the creation of steel using the oxygen-converter method. In the resulting compound, the proportion of manganese and silicon is extremely small.
Cast iron also has several varieties
:
- Half-hearted
. This cast iron has special properties, since part of the carbon in the composition is in the form of cementite, and the other part is in the form of graphite. - White
. Here the carbon is in the form of iron carbide. The name comes from the white hue of the fault. White cast iron is not used in its pure form, but is actively used in the creation of malleable cast iron. - Gray
. The cast at the fracture is silvery, which is why this cast iron is called gray. The scope of use of the material is quite wide, including because cast iron is easy to process with cutters. - High strength
. This variety can increase the strength of any material to which it is added. The material is obtained from gray cast iron and a small amount of magnesium. - Forgeable
. As with the high-strength variety, the base is gray cast iron. The annealing process helps increase ductility.
Other structural components in the iron-carbon system
In addition to components and phases, the system of iron-carbon alloys contains other structural components - pearlite and ledeburite.
Perlite
Pearlite is a eutectoid, a mechanical mixture of ferrite and cementite, obtained as a result of the decomposition of austenite when alloys are cooled below 727 ° C. With slow cooling, pearlite is present in all alloys with a carbon concentration from 0.02 to 6.67%. Under a microscope, perlite can appear either as plates or as grains - granular perlite. Its appearance, as well as its mechanical properties, depends on the cooling rate of the alloy and the type of heat treatment.
Ledeburite in steels
Ledeburite is a eutectic, a mechanical mixture of austenite and cementite, released from a liquid when alloys are cooled below 1147° C. The fundamental difference between the eutectic component and the eutectoid component is that the first is released from the liquid, and the second from the solid solution, in the case of iron-carbon alloys - from austenite . This structural component received its name in honor of the German metallurgist Ledebur.
Structure and properties
As the temperature increases, austenitic steels turn into a liquid solution with a certain percentage of iron and carbon. If the temperature of the solution exceeds the so-called liquidus line (about 1700 °C), the resulting melt becomes statically unstable. Its condition is assessed by two components: phase and structural.
For the first component, the main indicator is the state phase of the resulting mixture. It determines the condition of the metal according to the following indicators:
- solution of carbon in iron;
- number of different formations (ferrite itself, including high-temperature ferrite, austenite, cementite).
The structural component of a sample is defined as a homogeneous or quasi-homogeneous form. The general structure of the resulting ferrite is equiaxed crystals. In three-dimensional space, the ferrite phase lattice represents a body-centered cube. These crystals determine the hardness of ferrite and the ability of carbon to dissolve in it. Experience shows that at a temperature of 727 degrees only 0.02% of carbon dissolves in ferrite.
In addition, the main properties of ferrite include:
- has strong ferromagnetic properties (up to a temperature of 770 ° C - Curie point);
- is a heat-conducting element;
- good conductor of electric current;
- has increased plasticity.
The main disadvantages include low strength and insufficient hardness. The latter indicator depends on the size of the grain formed and is in the range from 65 to 130 HB.
Depending on the stage of ongoing transformations, the ferrite phase is in the following states:
- as the basis of the crystal lattice of the resulting alloy;
- second or excess state (located along the boundaries of the so-called pearlite formations);
- ferrite-graphite eutectoid element.
Each condition requires precise definition and identification of emerging transformations. The characteristics of the final product largely depend on them. The complete absence of ferrite formation or its insignificant content manifests itself with the formation of hot cracks. An overestimated content of this indicator reduces ductility, impact strength and anti-corrosion resistance.
Cast iron
Alloys on the iron-carbon diagram that contain more than 2.14% carbon are called cast irons. They are highly fragile. The cross section of such cast iron has a light tone, and therefore it is called white cast iron.
On the diagram this is point C, called the eutectic, with a corresponding carbon content of 4.3%. Crystallization produces a mixture of austenite and cementite, collectively called ledeburite. The phase composition is constant.
When the carbon concentration is less than 4.3% (hypoeutectic cast iron), austenite is released from the solution during crystallization. Next, C2 is isolated from it. And at 727° C, austenite turns into pearlite. The structural state of such cast iron is as follows: large areas of dark-colored pearlite.
In hypereutectic white cast iron (carbon more than 4.3%), upon cooling, structuring occurs with the formation of C1 crystals. Further transformations are carried out in the solid state. The structure is ledeburite, which is the background for fields of dark-toned pearlite. And large layers are Ts1.
Description
The carbon concentration in cementite—6.67% by weight—is the limit for iron-carbon alloys. Cementite is a metastable phase; the formation of a stable phase—graphite—is difficult in many cases. Cementite has an orthorhombic crystal lattice, is very hard and brittle, and weakly magnetic up to 210 °C.
Depending on the conditions of crystallization and subsequent processing, cementite can have different shapes - equiaxed grains, a network along grain boundaries, plates, as well as a Widmanstätt structure.
Cementite in different quantities, depending on the concentration, is present in iron-carbon alloys even at low carbon contents. Formed during the process of crystallization from molten cast iron. In steels it is released when austenite is cooled or martensite is heated. Cementite is a phase and structural component of iron-carbon alloys, an integral part of ledeburite, perlite, sorbite and troostite. Cementite is a representative of the so-called interstitial phases, compounds of transition metals with light metalloids. In the interstitial phases, the proportion of both covalent and metallic bonds is high.
Brinell hardness is more than 800 kg/mm2. Primary cementite crystallizes from a liquid alloy Secondary cementite - from austenite Tertiary cementite - from ferrite
This is interesting: Cementing steel at home with graphite and other methods - we analyze it thoroughly
conclusions
It is impossible to achieve absolute equilibrium, both physical and chemical, except under special laboratory conditions.
In practice, equilibrium can be close to absolute, but under certain conditions: a slow increase or decrease in the temperature of the alloy is sufficient, which will be maintained for a long time.
Sources
- https://FB.ru/article/340918/diagramma-jeleza-ugleroda-diagramma-sostoyaniya-sistemyi-jelezo-uglerod
- https://PokVorota3.ru/prokat/zhelezo-uglerod-2.html
- https://intehstroy-spb.ru/spravochnik/diagramma-sostoyaniya-zhelezo-uglerod-2.html
- https://NiceSpb.ru/materialy/diagramma-zhelezo.html
- https://pressadv.ru/stali/zhelezo-uglerod.html
- https://wiki2.org/ru/%D0%94%D0%B8%D0%B0%D0%B3%D1%80%D0%B0%D0%BC%D0%BC%D0%B0_%D1%81 %D0%BE%D1%81%D1%82%D0%BE%D1%8F%D0%BD%D0%B8%D1%8F_%D1%81%D0%BF%D0%BB%D0%B0%D0 %B2%D0%BE%D0%B2_%D0%B6%D0%B5%D0%BB%D0%B5%D0%B7%D0%BE-%D1%83%D0%B3%D0%BB%D0% B5%D1%80%D0%BE%D0%B4
- https://generator98.ru/raboty-so-stalyu/tablica-zhelezo-uglerod.html
Physics 8th grade. Melting and crystallization
Physics 8th grade Notes Melting Melting and crystallization. Specific heat of fusion.
Problems on the topic Thermal phenomena
The transition of a substance from solid to liquid is called melting.
Melting of crystalline bodies occurs only at a certain temperature.
The temperature at which a substance melts is called the melting point of the substance.
To carry out the melting process, the solid must first be heated to its melting point.
If a body is heated to the melting temperature and the heater is removed (stop supplying heat to the body), then melting does not occur.
To melt a body, two conditions must be met: 1. heat the body to the melting temperature 2. continue the transfer of heat
Melting point is an important thermal characteristic of a substance. Different substances have different melting points.
Melting metal
The transition of a substance from a liquid to a solid state is called solidification or crystallization.
For crystallization of a molten (liquid) body to begin, it must cool to a certain temperature.
The temperature at which a substance hardens (crystallizes) is called the solidification or crystallization temperature.
Graph of melting and solidification of crystalline solids.
Melting and solidification schedule
Experience shows that substances solidify at the same temperature at which they melt.
To carry out the solidification process, two conditions must be met: 1. cool the liquid to the solidification (melting) temperature 2. continue to remove heat until all the liquid has solidified.
A physical quantity showing how much heat must be imparted to a crystalline body weighing 1 kg in order to completely transform it into a liquid state at the melting point is called the specific heat of fusion.
The specific heat of fusion is denoted by the letter λ and measured in J/kg.
To calculate the amount of heat Q required to melt a crystalline body of mass m, taken at its melting point and normal atmospheric pressure,
you need to multiply the specific heat of fusion λ by the body mass:
where Q is the amount of heat, m is body mass.
The melting and crystallization temperatures for a given substance at constant external pressure are equal.
Crystallization
The amount of heat released during the crystallization of a substance, at a constant external pressure, is equal to the amount of heat received by this substance during melting.
Tasks
Evaporation and condensation
The abstract was compiled based on the theoretical material of the textbook “Physics 8th grade” by A.V. Peryshkin, “Physics 8th grade” A.V. Grachev.
Download abstract:
teoriya_8_plavleniekristallizacziya
Physics 8th grade. Quantity of heat. Specific heat. Fuel.
Physics 8th grade. Boiling. Condensation.
Physics 8th grade. Evaporation. Saturated steam. Air humidity.
Physics 8th grade. Thermal phenomena. Internal energy.