Each metal or alloy has unique properties, including its melting point. In this case, the object passes from one state to another, in a particular case, it becomes liquid from solid. To melt it, you need to apply heat to it and heat it until the desired temperature is reached. At the moment when the desired temperature point of a given alloy is reached, it may still remain in a solid state. As exposure continues, it begins to melt.
Mercury has the lowest melting point - it melts even at -39 °C, tungsten has the highest - 3422 °C. For alloys (steel and others) it is extremely difficult to determine the exact figure. It all depends on the ratio of the components in them. For alloys it is written as a numerical interval.
How the process works
Elements, whatever they are: gold, iron, cast iron, steel or any other, melt approximately the same. This occurs due to external or internal heating. External heating is carried out in a thermal furnace. For internal applications, resistive heating is used, passing an electric current or induction heating in a high-frequency electromagnetic field . The impact is approximately the same.
When heating occurs , the amplitude of thermal vibrations of molecules increases. Structural lattice defects appear , accompanied by the rupture of interatomic bonds. The period of lattice destruction and accumulation of defects is called melting.
Depending on the degree at which metals melt, they are divided into:
- low-melting - up to 600 °C: lead, zinc, tin;
- medium-melting - from 600 °C to 1600 °C: gold, copper, aluminum, cast iron, iron and most of all elements and compounds;
- refractory - from 1600 °C: chromium, tungsten, molybdenum, titanium.
Depending on what the maximum degree is, the melting apparatus is selected. It should be stronger the stronger the heating.
The second important value is the boiling degree. This is the parameter at which liquids begin to boil. As a rule, it is twice the melting point. These values are directly proportional to each other and are usually given at normal pressure.
If the pressure increases, the amount of melting also increases. If the pressure decreases, then it decreases.
Molybdenum
If you find out which metal is the most refractory, then, in addition to the indicated tungsten, you can also name molybdenum. Its melting point is 2623 °C. At the same time, it is quite hard, plastic and amenable to processing.
It is mainly used not in its pure form, but as an integral component of alloys. They, thanks to the presence of molybdenum, are significantly strengthened in wear resistance, heat resistance and anti-corrosion.
Some molybdenum compounds are used as technical lubricants. This metal is also an alloying material that simultaneously affects both strength and corrosion resistance, which is very rare.
Characteristics table
Metals and alloys are an indispensable basis for forging , foundry, jewelry and many other areas of production. Whatever the craftsman does ( gold jewelry , cast iron fences, steel knives or copper bracelets) , in order to work correctly, he needs to know the temperatures at which this or that element melts.
To find out this parameter, you need to refer to the table. In the table you can also find the boiling degree.
Among the most commonly used elements in everyday life, the melting point indicators are as follows:
- aluminum - 660 °C;
- copper melting point - 1083 °C;
- melting point of gold - 1063 °C;
- silver - 960 °C;
- tin - 232 °C. Tin is often used for soldering, since the temperature of a working soldering iron is exactly 250–400 degrees;
- lead - 327 °C;
- melting point of iron - 1539 °C;
- the melting point of steel (an alloy of iron and carbon) is from 1300 °C to 1500 °C. It varies depending on the saturation of the steel with components;
- melting point of cast iron (also an alloy of iron and carbon) - from 1100 °C to 1300 °C;
- mercury - -38.9 °C.
As is clear from this part of the table, the most fusible metal is mercury, which at positive temperatures is already in a liquid state.
The boiling point of all these elements is almost twice, and sometimes even higher than the melting point. For example, for gold it is 2660 °C, for aluminum - 2519 °C , for iron - 2900 °C, for copper - 2580 °C, for mercury - 356.73 °C.
For alloys such as steel, cast iron and other metals, the calculation is approximately the same and depends on the ratio of components in the alloy.
Read also: Crossbow pipe bender for copper pipes
The maximum boiling point of metals is rhenium - 5596 ° C. The highest boiling point is for the most refractory materials.
There are tables that also indicate the density of metals . The lightest metal is lithium, the heaviest is osmium. Osmium has a higher density than uranium and plutonium when viewed at room temperature. Light metals include: magnesium, aluminum, titanium. Heavy metals include most common metals: iron, copper, zinc, tin and many others. The last group is very heavy metals, these include: tungsten, gold, lead and others.
Another indicator found in the tables is the thermal conductivity of metals . Neptunium is the worst conductor of heat, and the best metal in terms of thermal conductivity is silver. Gold, steel, iron, cast iron and other elements are in the middle between these two extremes. Clear characteristics for each can be found in the required table.
Each metal or alloy has unique properties, including its melting point. In this case, the object passes from one state to another, in a particular case, it becomes liquid from solid. To melt it, you need to apply heat to it and heat it until the desired temperature is reached. At the moment when the desired temperature point of a given alloy is reached, it may still remain in a solid state. As exposure continues, it begins to melt.
Mercury has the lowest melting point - it melts even at -39 °C, tungsten has the highest - 3422 °C. For alloys (steel and others) it is extremely difficult to determine the exact figure. It all depends on the ratio of the components in them. For alloys it is written as a numerical interval.
Vanadium
Gray metal with a silvery sheen. It has a fairly high fusibility index (1920 °C). It is used mainly as a catalyst in many processes due to its inertness. It is used in the energy sector as a chemical current source, in the production of inorganic acids. It is not the pure metal that is of primary importance, but rather some of its compounds.
How the process works
Elements, whatever they are: gold, iron, cast iron, steel or any other, melt approximately the same. This occurs due to external or internal heating. External heating is carried out in a thermal furnace. For internal purposes, resistive heating is used, passing electric current or induction heating in a high-frequency electromagnetic field
. The impact is approximately the same.
When does heating occur
, the amplitude of thermal vibrations of molecules increases.
Structural lattice defects
appear , accompanied by the rupture of interatomic bonds. The period of lattice destruction and accumulation of defects is called melting.
Depending on the degree at which metals melt, they are divided into:
- low-melting - up to 600 °C: lead, zinc, tin;
- medium-melting – from 600 °C to 1600 °C: gold, copper, aluminum, cast iron, iron and most of all elements and compounds;
- refractory – from 1600 °C: chromium, tungsten, molybdenum, titanium.
Depending on what the maximum degree is, the melting apparatus is selected. It should be stronger the stronger the heating.
The second important value is the boiling degree. This is the parameter at which liquids begin to boil. As a rule, it is twice the melting point. These values are directly proportional to each other and are usually given at normal pressure.
If the pressure increases, the amount of melting also increases. If the pressure decreases, then it decreases.
Which metal doesn't heat up?
American physicists have discovered a metal that conducts electricity without heating up. We are talking about the metal vanadium dioxide (VO2). ... This material is known for its ability to transform from a dielectric to a conductive metal at a temperature of 67 degrees Celsius.
Interesting materials:
Where did they call 904 from? Why does 0 squared equal 1? Why don't British cats like to be held? Why does Excel remove leading zeros? Why do raspberries have no berries? Why is the oceanic crust thinner? Why are olives green? Why is the fur coat with the fur on the outside? Why does an owl's head turn? Why are there 20 cigarettes in a pack?
Characteristics table
Metals and alloys are an indispensable basis for forging
, foundry production, jewelry production and many other areas of production.
Whatever the craftsman does ( gold jewelry
, cast iron fences, steel knives or
copper bracelets)
, in order to work correctly, he needs to know the temperatures at which this or that element melts.
To find out this parameter, you need to refer to the table. In the table you can also find the boiling degree.
Among the most commonly used elements in everyday life, the melting point indicators are as follows:
- aluminum – 660 °C;
- copper melting point – 1083 °C;
- gold melting point – 1063 °C;
- silver – 960 °C;
- tin – 232 °C. Tin is often used for soldering, since the temperature of a working soldering iron is exactly 250–400 degrees;
- lead – 327 °C;
- melting point of iron – 1539 °C;
- the melting point of steel (an alloy of iron and carbon) is from 1300 °C to 1500 °C. It varies depending on the saturation of the steel with components;
- melting point of cast iron (also an alloy of iron and carbon) – from 1100 °C to 1300 °C;
- mercury – -38.9 °C.
As is clear from this part of the table, the most fusible metal is mercury, which at positive temperatures is already in a liquid state.
Read also: How to reduce vacuum cleaner speed
The boiling point of all these elements is almost twice, and sometimes even higher than the melting point. For example, for gold it is 2660 °C, for aluminum
- 2519 °C
, for iron - 2900 °C, for copper - 2580 °C, for mercury - 356.73 °C.
For alloys such as steel, cast iron and other metals, the calculation is approximately the same and depends on the ratio of components in the alloy.
The maximum boiling point of metals is rhenium – 5596
°C
. The highest boiling point is for the most refractory materials.
There are tables that also indicate the density of metals
.
The lightest metal is lithium, the heaviest is osmium. Osmium has a higher density than uranium
and plutonium when viewed at room temperature. Light metals include: magnesium, aluminum, titanium. Heavy metals include most common metals: iron, copper, zinc, tin and many others. The last group is very heavy metals, these include: tungsten, gold, lead and others.
Another indicator found in the tables is the thermal conductivity of metals
. Neptunium is the worst conductor of heat, and the best metal in terms of thermal conductivity is silver. Gold, steel, iron, cast iron and other elements are in the middle between these two extremes. Clear characteristics for each can be found in the required table.
Melting point, along with density, refers to the physical characteristics of metals
.
The melting point of a metal
is the temperature at which a metal changes from its normal solid state (except mercury) to a liquid state when heated.
atmospheric pressure does not affect
the melting temperature .
The melting point of metals ranges from -39 degrees Celsius to +3410 degrees
. For most metals, the melting point is high, however, some metals can be melted at home by heating on a regular burner (tin, lead).
Classification of metals by melting point
- Low-melting metals
, the melting point of which ranges
up to 600
degrees Celsius, for example
zinc, tin, bismuth
. - Medium-melting metals
that melt at temperatures
from 600 to 1600
degrees Celsius: such as
aluminum, copper, tin, iron
. - Refractory metals
, the melting point of which reaches
more than 1600
degrees Celsius -
tungsten, titanium, chromium
, etc. - - the only metal that is in a liquid state under normal conditions (normal atmospheric pressure, average ambient temperature). The melting point of mercury is about -39 degrees
Celsius.
Table of melting temperatures of metals and alloys
650
1000
| Metal | |
| Aluminum | 660,4 |
| Tungsten | 3420 |
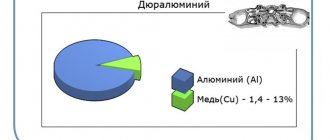
| Duralumin | |
| Iron | 1539 |
| Gold | 1063 |
| Iridium | 2447 |
| Potassium | 63,6 |
| Silicon | 1415 |
| Brass | |
| Low melting alloy | 60,5 |
| Magnesium | 650 |
| Copper | 1084,5 |
| Sodium | 97,8 |
| Nickel | 1455 |
| Tin | 231,9 |
| Platinum | 1769,3 |
| Mercury | –38,9 |
| Lead | 327,4 |
| Silver | 961,9 |
| Steel | 1300-1500 |
| Zinc | 419,5 |
| Cast iron | 1100-1300 |
When melting metal for the manufacture of metal castings, the choice of equipment, material for molding the metal, etc. depends on the melting temperature. It should also be remembered that when alloying the metal with other elements, the melting temperature most often decreases
.
Do not confuse the concepts of “metal melting point” and “metal boiling point” - for many metals these characteristics are significantly different: for example, silver melts at a temperature of 961 degrees Celsius, and boils only when the temperature reaches 2180 degrees.
The melting point of a metal is the minimum temperature at which it changes from solid to liquid. When melting, its volume practically does not change. Metals are classified by melting point depending on the degree of heating.
Melting point of steel
A table of melting temperature values for common grades of steel is presented. Steels for castings, structural, heat-resistant, carbon and other classes of steels are considered.
The melting point of steel ranges from 1350 to 1535°C.
The steels in the table are arranged in order of increasing melting point. Melting point of steel - table
| Steel | tmelt, °С | Steel | tmelt, °С |
| Steels for castings Х28Л and Х34Л | 1350 | Corrosion-resistant heat-resistant 12Х18Н9Т | 1425 |
| Structural steel 12Х18Н10Т | 1400 | Heat-resistant high-alloy 20Х23Н13 | 1440 |
| Heat-resistant high-alloy 20Х20Н14С2 | 1400 | Heat-resistant high-alloy 40Х10С2М | 1480 |
| Heat-resistant high-alloy 20Х25Н20С2 | 1400 | Corrosion-resistant steel X25S3N (EI261) | 1480 |
| Structural steel 12Х18Н10 | 1410 | Heat-resistant high-alloy 40Х9С2 (ESKH8) | 1480 |
| Corrosion-resistant heat-resistant 12Х18Н9 | 1410 | Corrosion-resistant ordinary 95Х18…15Х28 | 1500 |
| Heat-resistant steel Х20Н35 | 1410 | Corrosion-resistant heat-resistant 15Х25Т (EI439) | 1500 |
| Heat-resistant high-alloy 20Х23Н18 (ЭИ417) | 1415 | Carbon steels | 1535 |
Sources:
- Volkov A.I., Zharsky I.M. Large chemical reference book. - M: Soviet School, 2005. - 608 p.
- Kazantsev E.I. Industrial furnaces. Reference manual for calculations and design.
- Physical quantities. Directory. A. P. Babichev, N. A. Babushkina, A. M. Bratkovsky and others; Ed. I. S. Grigorieva, E. Z. Meilikhova. - M.: Energoatomizdat, 1991. - 1232 p.
Medium melting metals
Medium-melting metals begin to transform from solid to liquid at temperatures from 600°C to 1600°C. They are used to make slabs, reinforcements, blocks and other metal structures suitable for construction. This group of metals includes iron, copper, aluminum, and they are also part of many alloys. Copper is added to alloys of precious metals such as gold, silver, and platinum. 750 gold consists of 25% alloy metals, including copper, which gives it a reddish tint. The melting point of this material is 1084 °C. And aluminum begins to melt at a relatively low temperature of 660 degrees Celsius. This is a lightweight, ductile and inexpensive metal that does not oxidize or rust, therefore it is widely used in the manufacture of tableware. The melting point of iron is 1539 degrees. This is one of the most popular and affordable metals, its use is widespread in the construction and automotive industries. But due to the fact that iron is subject to corrosion, it must be additionally processed and covered with a protective layer of paint, drying oil, or prevent moisture from entering.
Read also: Internet cable plug
Tantalum
Metal, in its free form and under normal conditions, covered with an oxide film. It has a set of physical properties that allow it to be widespread and very important for humans. Its main characteristics are as follows:
- At temperatures above 1000 oC it becomes a superconductor.
- It is the most refractory metal after tungsten and rhenium. The melting point is 3017 °C.
- Absorbs gases perfectly.
- It is easy to work with as it can be rolled into sheets, foil and wire without much difficulty.
- It has good hardness and is not brittle, retains ductility.
- Very resistant to chemical agents (does not dissolve even in aqua regia).
Thanks to these characteristics, it has managed to gain popularity as the basis for many heat-resistant and acid-resistant, anti-corrosion alloys. Its numerous compounds are used in nuclear physics, electronics, and computational devices. Used as superconductors. Previously, tantalum was used as an element in incandescent lamps. Now tungsten has taken its place.
Refractory metals
The temperature of refractory metals is above 1600°C. These are tungsten, titanium, platinum, chromium and others. They are used as light sources, machine parts, lubricants, and in the nuclear industry. They are used to make wires, high-voltage wires, and are used to melt other metals with a lower melting point. Platinum begins to transition from solid to liquid at a temperature of 1769 degrees, and tungsten at a temperature of 3420°C.
Mercury is the only metal that is in a liquid state under normal conditions, namely, normal atmospheric pressure and average ambient temperature. The melting point of mercury is minus 39°C. This metal and its vapors are poisonous, so it is used only in closed containers or in laboratories. A common use of mercury is as a thermometer to measure body temperature.
Each metal and alloy has its own unique set of physical and chemical properties, not least of which is the melting point. The process itself means the transition of a body from one state of aggregation to another, in this case, from a solid crystalline state to a liquid one. To melt a metal, it is necessary to apply heat to it until the melting temperature is reached. With it, it can still remain in a solid state, but with further exposure and increased heat, the metal begins to melt. If the temperature is lowered, that is, some of the heat is removed, the element will harden.
The highest melting point among metals belongs to tungsten : it is 3422C o, the lowest is mercury: the element melts at -39C o. As a rule, it is not possible to determine the exact value for alloys: it can vary significantly depending on the percentage of components. They are usually written as a number interval.
Concept of temperature scale
Some non-metallic objects also have similar properties. The most common is water. A temperature scale was developed regarding the properties of the liquid that occupies a dominant position on Earth. The reference points are the temperature of changes in the aggregative states of water:
- Transformations from liquid to solid and vice versa are taken to be zero degrees.
- Boiling (vapor formation inside a liquid) at normal atmospheric pressure (760 mm Hg) is taken to be 100 ⁰C.
Attention! In addition to the Celsius scale, in practice temperature is measured in degrees Fahrenheit and on the absolute Kelvin scale. But when studying the properties of metal objects, other scales are used quite rarely.
How it happens
Melting of all metals occurs approximately the same way - using external or internal heating. The first is carried out in a thermal furnace; for the second, resistive heating is used by passing an electric current or induction heating in a high-frequency electromagnetic field. Both options affect the metal approximately equally.
As the temperature increases the amplitude of thermal vibrations of molecules , and structural defects in the lattice arise, expressed in the growth of dislocations, jumping of atoms and other disturbances. This is accompanied by the rupture of interatomic bonds and requires a certain amount of energy. At the same time, a quasi-liquid layer forms on the surface of the body. The period of lattice destruction and defect accumulation is called melting.
Metal separation
Depending on their melting point, metals are divided into:
- Low-melting: they need no more than 600C o. This is zinc, lead, hang, tin.
- Medium melting: melting point ranges from 600C to 1600C. These are gold, copper, aluminum, magnesium, iron, nickel and more than half of all elements.
- Refractory: temperatures above 1600C are required to make the metal liquid. These include chromium, tungsten, molybdenum, titanium.
Depending on the melting temperature, the melting apparatus is also selected . The higher the indicator, the stronger it should be. You can find out the temperature of the element you need from the table.
Another important quantity is the boiling point. This is the value at which the process of boiling liquids begins; it corresponds to the temperature of saturated steam that forms above the flat surface of the boiling liquid. It is usually almost twice the melting point.
Both values are usually given at normal pressure. directly proportional to each other .
Chrome and its alloys
One of the hardest metals, naturally bluish-white in color. Its melting point is lower than that of the elements considered so far, and is 1907 °C. However, it is still used everywhere in technology and industry, as it lends itself well to mechanical stress, is processed and molded.
Chromium is especially valuable as a coating. It is applied to products to give them a beautiful shine, protect against corrosion and increase wear resistance. The process is called chrome plating.
Chromium alloys are very popular. After all, even a small amount of this metal in the alloy significantly increases the hardness and resistance of the latter to impacts.