Iron is an element of the side subgroup of the eighth group of the fourth period of the periodic system of chemical elements of D.I. Mendeleev with atomic number 26. It is designated by the symbol Fe (lat. Ferrum). One of the most common metals in the earth's crust (second place after aluminum). Medium activity metal, reducing agent.
Main oxidation states – +2, +3
A simple substance, iron is a malleable silver-white metal with high chemical reactivity: iron quickly corrodes at high temperatures or high humidity in the air. Iron burns in pure oxygen, and in a finely dispersed state it spontaneously ignites in air.
STRUCTURE
Two modifications of the iron crystal lattice
Several polymorphic modifications have been established for iron, of which the high-temperature modification - γ-Fe (above 906°) forms a lattice of a face-centered cube of the Cu type (a0 = 3.63), and the low-temperature modification - the α-Fe lattice of a centered cube of the α-Fe type (a0 = 2.86). Depending on the heating temperature, iron can be found in three modifications, characterized by different crystal lattice structures:
- In the temperature range from the lowest to 910°C - a-ferrite (alpha ferrite), which has a crystal lattice structure in the form of a centered cube;
- In the temperature range from 910 to 1390°C - austenite, the crystal lattice of which has the structure of a face-centered cube;
- In the temperature range from 1390 to 1535°C (melting point) - d-ferrite (delta ferrite). The crystal lattice of d-ferrite is the same as that of a-ferrite. The only difference between them is the different (larger for d-ferrite) distances between the atoms.
When liquid iron is cooled, primary crystals (crystallization centers) appear simultaneously at many points in the cooled volume. With subsequent cooling, new crystalline cells are built around each center until the entire supply of liquid metal is exhausted. The result is a granular structure of the metal. Each grain has a crystal lattice with a certain direction of its axes. With subsequent cooling of solid iron, during the transitions of d-ferrite to austenite and austenite to a-ferrite, new crystallization centers may appear with a corresponding change in grain size
Composition and structure of iron
Iron is a typical metal, and it is chemically active. The substance reacts at normal temperature, and heating or increasing humidity significantly increases its reactivity. Iron corrodes in air, burns in an atmosphere of pure oxygen, and in the form of fine dust can ignite in air.
Pure iron is inherently malleable, but the metal is very rare in this form. In fact, iron means an alloy with small proportions of impurities - up to 0.8%, which is characterized by the softness and malleability of a pure substance. Alloys with carbon - steel, cast iron, stainless steel - are important for the national economy.
Iron is characterized by polymorphism: there are as many as 4 modifications, differing in structure and lattice parameters:
- α-Fe - exists from zero to +769 C. It has a body-centered cubic lattice and is ferromagnetic, that is, it retains magnetization in the absence of an external magnetic field. +769 C – Curie point for metal;
- from +769 to +917 C β-Fe . It differs from the α phase only in lattice parameters. Almost all physical properties are preserved with the exception of magnetic ones: iron becomes paramagnetic, that is, it loses the ability to magnetize and is drawn into a magnetic field. Metallurgy does not consider the β-phase as a separate modification. Since the transition does not affect significant physical characteristics;
- in the range from 917 to 1394 C there is a γ modification , which is characterized by a face-centered cubic lattice;
- the δ phase appears , which is characterized by a body-centered cubic lattice.
Crystallographic characteristics
Cubic system
Class hexoctahedral
Crystal structure of native iron – Body-centered cubic lattice (for low-temperature modification)
Form of being in nature
The appearance of crystals. Only microscopically small ferrite crystals are known.
Twins in native iron at (111) with a fusion plane (211), often repeated.
Aggregates.
Grains, scales, wire-like sticks, curved ribbons, impregnations in rocks, sometimes large continuous deposits weighing up to several tons (ferrite), often intergrown with cohenite.
PROPERTIES
Iron ore
In its pure form under normal conditions it is a solid. It has a silver-gray color and a pronounced metallic luster. The mechanical properties of iron include its level of hardness on the Mohs scale. It is equal to four (average). Iron has good electrical and thermal conductivity. The last feature can be felt by touching an iron object in a cold room. Because this material conducts heat quickly, it removes most of it from your skin in a short period of time, which is why you feel cold. If you touch, for example, wood, you will notice that its thermal conductivity is much lower. The physical properties of iron include its melting and boiling points. The first is 1539 degrees Celsius, the second is 2860 degrees Celsius. We can conclude that the characteristic properties of iron are good ductility and fusibility. But that's not all. Also, the physical properties of iron include its ferromagnetism. What it is? Iron, whose magnetic properties we can observe in practical examples every day, is the only metal that has such a unique distinctive feature. This is explained by the fact that this material is capable of magnetization under the influence of a magnetic field. And after the end of the action of the latter, the iron, the magnetic properties of which have just been formed, remains a magnet for a long time. This phenomenon can be explained by the fact that in the structure of this metal there are many free electrons that are able to move.
What is iron?
From an industrial point of view, iron is the main building material in the world. Its use is everywhere - from door handles to interplanetary rocket engines. The reason for this popularity is simple - the abundance and low cost of raw materials, because iron is in second place in terms of its share in the earth’s crust after aluminum. Symbolic designation of iron "Fe"? and its serial number according to Mendeleev is 26.
Important: do not confuse steel and iron - these are completely different terms. Iron is a pure chemical element, and steel is an alloy of ferum and carbon.
Sometimes iron in the industry is called a highly concentrated alloy with an impurity content of less than 0.7% of the total mass. In this case, the physical properties of the substance are almost not lost. Most iron-based alloys belong to the group of ferrous metals.
| Benefits of Iron | Disadvantages of metal |
| Preservation of elastic properties while increasing the strength of the metal - this is when we are talking about highly concentrated iron alloys. | Weak resistance to corrosion. The problem can be corrected, but production costs increase. |
| The abundance of ferrites makes it possible to produce iron-based materials for any household and industrial purposes. Moreover, a change in properties occurs even with minimal inclusions of impurities. | Due to the accumulation of electricity, iron is susceptible to electrochemical corrosion. For this reason, iron parts must be protected with protectors, cathodes and other protective equipment. |
| Easily machined, which increases variability in the shapes and types of products. | The specific gravity of pure iron makes structures made from it extremely heavy. |
| The magnetic properties of the metal make it possible to produce magnetic drives from it. The high malleability of iron allows it to be made into many decorative elements. |
In its pure form, iron has good ductility, making it easy to forge but difficult to cast. The structure of the metal is 5 phases, each of which has its own crystal structure and lattice parameters. More details in the table below.
| Phase | Peculiarities |
| α | Lattice type – cubic, body-centered shape. Phase stability – up to 770 degrees Celsius. Physical feature – ferromagnetic properties. |
| β | The existence of the phase occurs at temperatures ranging from 770 to 918 degrees Celsius. Complete preservation of physical and chemical properties, except magnetization. Iron becomes paramagnetic. The structure is similar to the previous phase, but the parameters of the lattice are slightly different. |
| γ | The temperature limits of existence are from 918 degrees to 1395 degrees. The key difference is the cubic + face-centric lattice. |
| δ | There is only a lower temperature limit of 1395 degrees. There is no upper limit. The type of lattice structure is volume-centric. |
| ε | The phase does not have clear temperature boundaries, but a necessary condition for its existence is high pressure + the connection of alloying components to pure iron. The lattice type is hexagonal with a dense arrangement of crystals. |
The physical properties of iron directly depend on the purity of the substance. In addition to useful alloying components, which have a positive effect on the properties of the metal, there are also negative elements that can worsen its characteristics. Examples of these could be sulfur and phosphorus, which reduce ductility, in return not providing the alloy with any of the positive properties.
Basic characteristics of iron:
- variable density depending on the residence phase of the metal. The general range is from 7.4 to 7.9 grams/cubic centimeter;
- The resistance of iron in its pure form is low. The tensile strength of ordinary technical iron is 299 MPa, but if we are talking about high-speed steel, then the tensile strength increases to a value of 2.7 GPa;
- pure iron on the Mohs scale is rated at 4 points;
- the conductivity of pure ferum is lower than that of aluminum/copper – 9.7*10^(-8);
- metal can be forged, but in its pure form cannot be cast;
- low toxicity, but not biologically inert.
The absorption of iron by the human body is only ½ of what is received, which makes the metal less dangerous for humans than other elements of the metal group. The main harm to the environment is not caused by iron in its pure form, but by waste during production - gases and slags released. More details about production, thermal characteristics and areas of application will be discussed below.
10 strongest metals in the world
Melting point table
It is important for anyone involved in the metallurgical industry, whether a welder, foundry worker, smelter or jeweler, to know the temperatures at which the materials they work with melt. The table below shows the melting points of the most common substances.
Table of melting temperatures of metals and alloys
| Name | T pl, °C |
| Aluminum | 660,4 |
| Copper | 1084,5 |
| Tin | 231,9 |
| Zinc | 419,5 |
| Tungsten | 3420 |
| Nickel | 1455 |
| Silver | 960 |
| Gold | 1064,4 |
| Platinum | 1768 |
| Titanium | 1668 |
| Duralumin | 650 |
| Carbon steel | 1100−1500 |
| Cast iron | 1110−1400 |
| Iron | 1539 |
| Mercury | -38,9 |
| Cupronickel | 1170 |
| Zirconium | 3530 |
| Silicon | 1414 |
| Nichrome | 1400 |
| Bismuth | 271,4 |
| Germanium | 938,2 |
| Tin | 1300−1500 |
| Bronze | 930−1140 |
| Cobalt | 1494 |
| Potassium | 63 |
| Sodium | 93,8 |
| Brass | 1000 |
| Magnesium | 650 |
| Manganese | 1246 |
| Chromium | 2130 |
| Molybdenum | 2890 |
| Lead | 327,4 |
| Beryllium | 1287 |
| Will win | 3150 |
| Fechral | 1460 |
| Antimony | 630,6 |
| titanium carbide | 3150 |
| zirconium carbide | 3530 |
| Gallium | 29,76 |
In addition to the melting table, there are many other supporting materials. For example, the answer to the question what is the boiling point of iron lies in the table of boiling substances. In addition to boiling, metals have a number of other physical properties, such as strength.
Melting iron
The melting point of a metal is the minimum temperature at which it changes from solid to liquid. At the same time, it remains practically unchanged in volume.
Iron is part of the group of medium-melting metals. Iron begins to turn into a liquid state only at a temperature of 1539 degrees. It is one of the most common metals used in industry, especially in the automotive industry. However, iron is susceptible to corrosion, that is, rust, so it requires special surface treatment. It must be coated with paint or drying oil, and moisture must not be allowed to enter.
Melting point of iron
The melting point of chemically pure iron is 1539 o C. Technically pure iron obtained as a result of oxidative refining contains a certain amount of oxygen dissolved in the metal. For this reason, its melting point drops to 1530 o C.
The melting point of steel is always lower than the melting point of iron due to the presence of impurities in it. Metals dissolved in iron (Mn, Cr, Ni. Co, Mo, V, etc.) lower the melting point of the metal by 1 - 3 o C per 1% of the introduced element, and elements from the group of metalloids (C, O, S, P and etc.) at 30 – 80 o C.
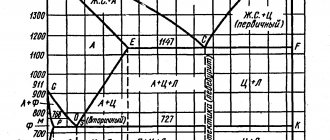
Over most of the total duration of the melt, the melting temperature of the metal changes mainly as a result of changes in carbon content. At a carbon concentration of 0.1 - 1.2%, which is typical for finishing smelting in steel-smelting units, the melting temperature of the metal can be estimated with sufficient accuracy for practical purposes from the equation
Heat of fusion of iron
The heat of fusion of iron is 15200 J/mol or 271.7 kJ/kg.
Boiling point of iron
The boiling point of iron in recent publications is given as 2735 o C. However, research results have been published according to which the boiling point of iron is much higher (up to 3230 o C).
Heat of vaporization of iron
The heat of evaporation of iron is 352.5 kJ/mol or 6300 kJ/kg.
Iron Density
The density of iron at 1550 - 1650 o C is 6700 - 6800 kg/m 3. At the crystallization temperature, the density of the liquid metal is close to 6850 kg/m3. The density of solid iron at the crystallization temperature is 7450 kg/m3, at room temperature - 7800 kg/m3.
Of the common impurities, carbon and silicon have the greatest influence on the density of iron melts, reducing it. Therefore, liquid cast iron of ordinary composition has a density of 6200 - 6400 kg/m 3, solid at room temperature - 7000 - 7200 kg/m 3.
Sources:
- https://pressadv.ru/materialy/temperatura-kipeniya-zheleza-otvet.html
- https://chem.ru/zhelezo.html
- https://co-vally.ru/stanki/zhelezo-vikipediya-metall.html
- https://spk-kovka.ru/materialy/1-kakova-temperatura-kipeniya-zheleza.html
- https://wikimetall.ru/spravochnik/kakova-temperatura-kipenija-zheleza.html
RESERVES AND PRODUCTION
Iron is one of the most common elements in the solar system, especially on the terrestrial planets, in particular on Earth. A significant part of the iron of the terrestrial planets is located in the cores of the planets, where its content is estimated to be about 90%. The iron content in the earth's crust is 5%, and in the mantle about 12%.
Iron
Iron is quite widespread in the earth's crust - it accounts for about 4.1% of the mass of the earth's crust (4th place among all elements, 2nd among metals). In the mantle and crust, iron is concentrated mainly in silicates, while its content is significant in basic and ultrabasic rocks, and low in acidic and intermediate rocks. A large number of ores and minerals containing iron are known. Of greatest practical importance are red iron ore (hematite, Fe2O3; contains up to 70% Fe), magnetic iron ore (magnetite, FeFe2O4, Fe3O4; contains 72.4% Fe), brown iron ore or limonite (goethite and hydrogoethite, respectively FeOOH and FeOOH nH2O ). Goethite and hydrogoethite are most often found in weathering crusts, forming so-called “iron hats”, the thickness of which reaches several hundred meters. They can also be of sedimentary origin, falling out of colloidal solutions in lakes or coastal areas of the seas. In this case, oolitic, or legume, iron ores are formed. Vivianite Fe3(PO4)2·8H2O is often found in them, forming black elongated crystals and radial aggregates. The iron content in sea water is 1·10−5-1·10−8%. In industry, iron is obtained from iron ore, mainly from hematite (Fe2O3) and magnetite (FeO·Fe2O3). There are various ways to extract iron from ores. The most common is the domain process. The first stage of production is the reduction of iron with carbon in a blast furnace at a temperature of 2000 °C. In a blast furnace, carbon in the form of coke, iron ore in the form of agglomerate or pellets, and flux (such as limestone) are fed from above, and are met by a stream of forced hot air from below. In addition to the blast furnace process, the process of direct iron production is common. In this case, pre-crushed ore is mixed with special clay, forming pellets. The pellets are fired and treated in a shaft furnace with hot methane conversion products, which contain hydrogen. Hydrogen easily reduces iron without contaminating the iron with impurities such as sulfur and phosphorus, which are common impurities in coal. Iron is obtained in solid form and is subsequently melted in electric furnaces. Chemically pure iron is obtained by electrolysis of solutions of its salts.
Properties and characteristics of iron
Iron is a fairly light, moderately refractory metal, silver-gray in color. Reacts easily with dilute acids and is therefore considered a medium activity element. In dry air, the metal is gradually covered with an oxide film, which prevents further reaction.
But at the slightest humidity, instead of a film, rust appears - loose and heterogeneous in composition. Rust does not prevent further corrosion of iron. However, the physical properties of the metal, and, most importantly, its alloys with carbon, are such that, despite the low corrosion resistance, the use of iron is more than justified.
Chemical composition
Telluric iron contains impurities of nickel (Ni) 0.6-2%, cobalt (Co) up to 0.3%, copper (Cu) up to 0.4%, platinum (Pt) up to 0.1%, carbon. Native iron usually contains Ni in solid solution. The composition of individual varieties has not been precisely established; the analyzes are mostly old, performed on material not verified by mineragraphic and x-ray studies. Minor admixtures of Co, Cu, S, C, Mn, P, Pt, As, Ge have been identified, partly associated, apparently, with a mechanical admixture of cohenite; the presence of gas inclusions (CO and CO2) was noted.
Varieties
Ferrite (Vernadsky, 1912) is the purest, almost Ni-free native iron. Awaruite - awaruite (Skei, 1885) 6 -(Ni, Fe). Native nickel-iron with a high Ni content (Ni: Fe from 4: 1 to 2: 1). Hardness. 5. Density 8.1. It resembles polyxene in luster and color. In reflected light, pure white or light cream, isotropic, highly reflective. Named for its location in Avarua Bay (New Zealand), where it is associated with gold, platinum, cassiterite, chromite, and magnetite. It is found as a secondary mineral in peridotites that have undergone serpentinization, serpentinites, trachytes, and quartz porphyries. Varieties of nickel-iron of terrestrial origin close to or identical to awaruite, found in placers and in serpentinized peridotites, are described under the names: josephinite (Melville, 1892), souesite-souesite (Hofman, 1905), octibbehite (Taylor, 1905). 1857), catarinite - satarinite (Damur, 1877). Under the name bobrovkite (Vysotsky, 1913), nickel-iron (iron-nickel) is described, found in the form of fine-scaled grains together with platinum (polyxene) in the placers of M. Bobrovka (Ural). Contains Ni 71.93, Fe 28.07, as well as Co, Mn.
ORIGIN
Native iron
Origin telluric (terrestrial) iron is rarely found in basalt lavas (Uifak, Disko Island, off the western coast of Greenland, near Kassel, Germany). At both locations, pyrrhotite (Fe1-xS) and cohenite (Fe3C) are associated with it, which is explained by both the reduction by carbon (including from the host rocks) and the decomposition of carbonyl complexes such as Fe(CO)n. In microscopic grains, it has more than once been established in altered (serpentinized) ultrabasic rocks, also in paragenesis with pyrrhotite, sometimes with magnetite, due to which it arises during reduction reactions. Very rarely found in the oxidation zone of ore deposits, during the formation of swamp ores. Findings have been recorded in sedimentary rocks associated with the reduction of iron compounds with hydrogen and hydrocarbons. Almost pure iron was found in lunar soil, which is associated with both meteorite falls and magmatic processes. Finally, two classes of meteorites - stony-iron and iron - contain natural iron alloys as a rock-forming component.
Methods for obtaining metal
There are several ways to get iron:
- Direct methods. This is the production of sponge iron in shaft and tunnel furnaces. Production of iron dough in rotary kilns. It is possible to obtain iron in fluidized bed reactors and the chemical-thermal method.
- The blast furnace process is a common method. Iron ore and flux are reduced by carbon from coke, resulting in cast iron. If necessary, impurities (phosphorus, sulfur) and excess carbon are removed from cast iron in open-hearth furnaces or converters. Alloy steel is produced in electric furnaces (ESF).
- Chemically pure iron can be obtained from a solution of its salts using electrolysis.
B. Meteoric iron
Kamacite – Kamacite – nickel iron (6-9% Ni). The name kamacite comes from the Greek - beam, rod (Reichenbach, 1861),
A synonym for kamacite is beam iron, taenite is band iron (Reichenbach, 1861), edmonsonite (Flight, 1882). Plessite (Reichenbach, 1861) is a fine mixture of kamacite and taenite.
Taenite – Taenite – nickel-iron (up to 48% Ni), taenite – from Taivia - ribbon, strip (Reichenbach, 1861).
For taenite of the Fe2Ni composition, the following names have been proposed: nikdiferrite (Chirvinsky, 1928), orthotenite (Badhu, 1936) and chirvinite (Astapovich, 1950); a compound of this composition is established in the Fe-Ni system. Metakamacite is a metastable a-modification of pickled iron, and, in addition, a granular variety of plessite (Owen, 1940). Metatenite is taenite with an admixture of kamacite (Badhyo, 1936).
Native iron of cosmic origin makes up the mass of iron meteorites. Found in most stony meteorites. Forms:
a) a solid mass of a meteorite;
b) a spongy mass in which grains of olivine or other silicates are immersed;
c) grains and scales scattered throughout the meteorite;
d) separate crystalline individuals with numerous twin plates. Kamacite and taenite are always closely intergrown. Iron meteorites from the octahedrite group are characterized by systems of intersecting stripes, which are called Widmanstätten figures: individual stripes consist of kamacite with taenite rims, with plessite between the intersecting stripes. Widmanstätten figures arise as a result of the decomposition of a solid solution of γ-iron and nickel. In intergrowths of kamacite and taenite, the plane of the rhombic dodecahedron (110) of kamacite is parallel to the plane of the octahedron (111) of taenite, which is explained by their structural similarity.
APPLICATION
Iron ring
Iron is one of the most used metals, accounting for up to 95% of global metallurgical production. Iron is the main component of steels and cast irons - the most important structural materials. Iron can be part of alloys based on other metals - for example, nickel. Magnetic iron oxide (magnetite) is an important material in the production of long-term computer memory devices: hard drives, floppy disks, etc. Ultrafine magnetite powder is used in many black and white laser printers mixed with polymer granules as a toner. This uses both the black color of the magnetite and its ability to stick to the magnetized transfer roller. The unique ferromagnetic properties of a number of iron-based alloys contribute to their widespread use in electrical engineering for magnetic cores of transformers and electric motors. Iron(III) chloride (ferric chloride) is used in amateur radio practice for etching printed circuit boards. Ferrous sulfate heptate (ferrous sulfate) mixed with copper sulfate is used to combat harmful fungi in gardening and construction. Iron is used as an anode in iron-nickel batteries and iron-air batteries. Aqueous solutions of ferrous and ferric chlorides, as well as its sulfates, are used as coagulants in the purification processes of natural and waste water in the water treatment of industrial enterprises.
Iron – Fe
| Molecular weight | 55.85 g/mol |
| origin of name | Possibly Anglo-Saxon origin |
| IMA status | valid, first described before 1959 (before IMA) |
Extraction of iron from minerals
There are several ores containing iron. However, the following are mainly used as raw materials for iron production in industry:
- magnesite ore;
- goethite ore;
- hematite ore.
And also the following types of ore are often found:
- lellingitis;
- siderite;
- marcasite;
- ilmenite;
- is violent.
There is also a mineral called melanterite . It is used primarily in the pharmaceutical industry. It consists of green, fragile crystals with a glassy sheen. Medicines containing ferum are produced from it.
The main deposit of this metal is South America, namely Brazil.
History of discovery
Everyone remembers the “Iron Age” from school. This is the period in history when man first learned to extract this metal from ore. The Iron Age spans the period from the 9th to the 7th century BC. This metal had a huge influence on the development of people of that time. According to its characteristics, it has replaced mixtures of non-ferrous metals. Tools, weapons, armor, materials for construction and much more were made from it. Gradually, blacksmiths began to mix it with other metals to create new materials. This is how new alloys appeared.
PHYSICAL PROPERTIES
| Mineral color | iron black |
| Stroke color | grey |
| Transparency | opaque |
| Shine | metal |
| Cleavage | imperfect by {001} |
| Hardness (Mohs scale) | 4,5 |
| Kink | hackly |
| Strength | malleable |
| Density (measured) | 7.3 – 7.87 g/cm3 |
| Radioactivity (GRapi) | |
| Magnetism | ferromagnet |
Strength of metals
In addition to the ability to transition from a solid to a liquid state, one of the important properties of a material is its strength - the ability of a solid body to resist destruction and irreversible changes in shape. The main indicator of strength is the resistance that occurs when a pre-annealed workpiece breaks. The concept of strength does not apply to mercury because it is in a liquid state. The designation of strength is adopted in MPa - Mega Pascals.
There are the following strength groups of metals:
- Fragile. Their resistance does not exceed 50MPa. These include tin, lead, soft-alkaline metals
- Durable, 50−500 MPa. Copper, aluminum, iron, titanium. Materials of this group are the basis of many structural alloys.
- High strength, over 500 MPa. For example, molybdenum and tungsten.
Metal strength table
| Metal | Resistance, MPa |
| Copper | 200−250 |
| Silver | 150 |
| Tin | 27 |
| Gold | 120 |
| Lead | 18 |
| Zinc | 120−140 |
| Magnesium | 120−200 |
| Iron | 200−300 |
| Aluminum | 120 |
| Titanium | 580 |