Story
One of the most famous places where bronze items were found was located in the Kuban River area. In this place, archaeologist Nikolai Veselovsky in 1897 excavated the so-called Maykop culture, which existed in the second half of the 4th millennium BC.
Bronze artifacts found in the Maikop burial mounds were made mainly from an alloy of copper and arsenic, so it is believed that historically these alloys, called arsenic bronzes, .
It was in no way inferior in its properties to alloys of copper with tin or lead, and even surpassed them in a number of characteristics . It was widely used in various areas of human activity of those times, from the manufacture of critical parts to jewelry.
Bronze composition
Bronze is an alloy of copper with metals such as tin, aluminum, lead, beryllium, and non-metals arsenic, silicon and phosphorus. In addition, such alloys can be additionally alloyed with phosphorus, zinc, manganese, iron and nickel.
The composition of bronze depends on the grade of the alloy and is indicated in its designation. For example, the composition of an alloy labeled BrAMts7−1 includes 7% aluminum, 1% manganese and 92% copper .
Thus, the main component of this metal is copper (from 35% to 90% and above). The second component can be either arsenic, or tin or beryllium, lead, aluminum, silicon and other components. To impart special properties, additional components can be added - zinc, iron, nickel, manganese, phosphorus and others.
Production of material
Bronze is obtained from pure metals or alloys in ingots. The second is more common, as it is cheaper and allows you to get any cast bronze.
- The first stage is the extraction of copper and tin from the deposits. Tin is found in cassiterites, stannines, and so on. Copper is more common: it is mined from native copper and many minerals - chalcopyrite, bornite, chalcocite. The metal is isolated in several different ways, of which pyrometallurgical, that is, oxidative annealing and fire refining, is the most common.
- Then the composition of the charge is calculated: it depends on the composition of the future product and on the production method - from secondary alloys, from metal and secondary alloys, and so on.
- Melting itself, for example from pigs, includes several stages:
- loading - the material is loaded into dried and heated graphite-carboride or graphite-chamotte crucibles. Electric and electric arc furnaces are best suited as they allow melting to be carried out as quickly as possible. This is important because when melting metals, there is a high probability of gases being absorbed into the melt;
- melting - first of all, copper is melted, and then various components are added that improve the mechanical properties of the alloy, and basic alloying additives;
- overheating - the melt is heated to 1200 C under a layer of charcoal. If the source metals are contaminated, liquid salt fluxes are used;
- degassing - the melt is cleaned of gas impurities by blowing with argon or nitrogen.
- Castings are obtained from the finished alloy. Most often, gating systems are used for this. Casting is done in clay or metal molds. It is possible to produce castings using centrifugal casting.
In 2016, there was an increase in prices for copper - up to 4%, and tin - up to 10.3%. Accordingly, the cost of products made from bronze and bronze scrap increases. The latter in October had a cost from 190 to 210 rubles. per kg.
The price of products - rod, casting, sheet - depends on the composition of the alloy. So, a rod of different brands can be purchased for both 308 and 803 rubles. per kg.
Next we touch on the marking and use of bronze.
Features of bronze and properties
The main properties of all bronze alloys are ductility and hardness. Depending on the ratio of the main and additional components, a wide variety of new properties can be obtained. In addition, the amount of copper in the alloy determines its color.
So, golden bronze will be obtained if the alloy contains about 85% copper, and if its amount is reduced to 50%, an alloy will be obtained that has a silvery color. Reducing the amount of copper to 35% and below will lead to the output of gray and even black bronze, and increasing the amount of copper to 90% and above will lead to the formation of red bronze.
One of the old brands of bronze alloys is bell bronze , which is still used today for casting bells. It contains 20% tin and 80% copper. Its disadvantage is increased fragility due to the high tin content in the alloy.
As mentioned above, the most commonly used are alloys of copper and tin with the addition of a small amount of other components. The widespread use of such alloys is due, first of all, to historical reasons that led to the displacement of arsenic bronze from production.
Such reasons are the following:
- the development over many centuries of deposits of tennantite and other faded ores rich in copper and arsenic. Such ores were most convenient for the production of arsenic bronze, since they did not lie very deep, which made the production process cheaper compared to other sources of copper and arsenic;
- the high toxicity of the production of such bronze, caused by the presence of arsenic in the deposits, which inevitably led to loss of health and further ability to work for experienced metallurgists and blacksmiths;
- unsuitability of metallurgical defects and broken products made of arsenic bronze for further remelting into high-quality metal. At best, such products were used to make jewelry or non-essential parts.
The alloys of copper and tin that replaced arsenic bronzes, although they were more expensive to produce, were economically preferable , since the development of horse-drawn transport and the resulting establishment of trade relations between cities and countries led to an increase in the import of non-arsenic bronze.
Types of bronze and characteristics
The development of large-scale industrial production generally led to the fact that tin bronzes became almost the most widespread type of bronze. And only in the last hundred years this type began to be replaced by copper alloys with tin substitutes, such as aluminum, silicon and, especially, beryllium bronzes.
Thus, the following types exist:
- tin-free. It includes bronze, in which the second components are aluminum, silicon, beryllium and other metals and non-metals. Each of these components gives it special properties. For example, aluminum gives the alloy increased anti-friction properties and high corrosion resistance, beryllium increases strength and hardness, and silicon and zinc improve its fluidity and abrasion resistance;
- tin. A copper-tin alloy in which copper predominates. It is one of the first to be mastered by man. It has high hardness and strength compared to pure copper, and is also more fusible. In such alloys, tin is always the second most abundant after copper and the main alloying component.
The third in quantity are additional components such as arsenic, zinc and lead . Due to its very low shrinkage, this metal is mainly intended for casting, as it is difficult to work with pressure, cutting and sharpening. Even the tendency to segregation and low fluidity do not prevent the use of this alloy for the manufacture of configurationally complex castings, including in artistic casting.
Bronze with the addition of zinc is called “Admiralty ” and is used for the manufacture of parts that have frequent or constant contact with sea water (shipbuilding). This feature is due to the fact that zinc gives the alloy increased corrosion resistance in the specified environment.
However, to make bronze resistant to corrosion in salty seawater, it is increasingly being enriched with aluminum and nickel . Such alloys, often called “marine”, are used to manufacture elements of oil platforms operating on sea and ocean shelves.
To give bronze additional characteristics, of phosphorus, silver, zinc, arsenic, manganese and other components are alloyed into it Thus, adding a small amount of silver increases the electrical conductivity of bronze and makes it comparable to the electrical conductivity of copper.
Structure and composition
- The properties of the alloy - from color to fluidity in molten form - depend on two main factors: the qualitative composition of bronze and its structure. A qualitative composition is a set of metals or non-metals involved in creating an alloy in a tangible, significant amount. Moreover, the latter is determined not by the actual mass or volume of the substance, but by the severity of the properties that it creates. The addition of only 0.25% silver significantly increases electrical conductivity, and to obtain anti-friction properties you need to add at least 4% silicon.
The composition is indicated on the labeling, which allows you to choose the right material for certain jobs.
- The second factor is less known - the structure of the alloy or solid solution . The fact is that only 15.8% of tin can dissolve in copper, while in the alloy there can be noticeably more of it. This causes the appearance of alloys with different phase structures. Single-phase - the proportion of tin does not exceed 6–8%; there is only one α-phase. This composition is characterized by elasticity and high malleability. Moreover, bronze with a tin content of up to 2% can be forged in the cold without heating, and with a metal content of up to 8% - with heating.
- Two-phase - when the tin fraction exceeds 15%, that is, the maximum solubility, 2 phases appear in the solid solution. At the same time, such a quality as malleability completely disappears, and the alloy begins to gain hardness and some fragility. This alloy is used for casting.
Additional elements that influence the properties have little effect on the phase composition.
Nickel-containing tin bronzes
The addition of nickel to tin bronze gives the alloy such properties as significantly high strength characteristics, excellent ductility, good deformability, and resistance to corrosion processes. Due to the peculiarities of their structure, tin bronzes with nickel are well hardened by temperature exposure (hardening and subsequent aging).
A distinctive feature of these tin bronzes is their fatigue strength under corrosive conditions. This indicator increases when the tin content in the alloy is within 4 percent. When this threshold is exceeded, the increase in the characteristic under consideration slows down.
Application area
Bronze alloys, due to the diversity of their properties, find a wide variety of applications.
- The most famous is the material for sculptures and many decorative items: figurines, ashtrays, lamps, grilles, railing decorations and more. Casting bronze allows you to obtain the most complex castings that literally depict the pores of the skin.
- In jewelry, the material is used much less frequently, although it used to be almost the basis of women's jewelry.
- Bronze fittings - overhead hinges, locks, handles, taps, mixers and even plumbing fixtures. The alloy provides not only exceptional durability and corrosion resistance of objects, but also allows you to turn them into an elegant decorative element.
- Many parts are made from cast bronze of different compositions - gears, bushings, seals, parts of equipment designed to work under water.
- Deformable bronzes are used in high-precision technology.
- Other types of alloys are used in areas where the usual tin bronze is not used. For example, beryllium bronze has much higher thermal and electrical conductivity, and therefore is actively used in electrical engineering.
The alloy performs excellently under conditions of variable dynamic loads. Therefore, parts of aircraft navigation instruments, car circuits, etc. are made from beryllium bronze.
- Another well-known application is fittings of various kinds. For more active use in water supply, bronze is too expensive a material, however, the most critical components, as well as numerous fasteners, are made from a copper alloy, since it is extremely resistant to corrosion and inhibits bacterial activity.
Bronze is the oldest and most famous alloy in human history. The diversity of its composition and properties ensures that it is widely used today.
This video will show you how to clean bronze items:
Advantages and disadvantages
Bronze has many positive qualities. Among them:
- variety of properties and, consequently, areas of application;
- the ability to create options for various processing methods (casting or deformation) depending on needs;
- slight shrinkage (0.5 - 1.5%);
- the possibility of repeated processing without loss of properties, that is, bronze can be processed;
- high resistance to chemical influences of the environment (water, air, acids);
- greater elasticity of many options.
The main disadvantage is the high cost of some brands, for example, tin bronze. Types of other composition, such as aluminum alloy, are much cheaper. Thus, the cost of the materials under consideration is largely determined by the alloying elements included in their composition.
Marking
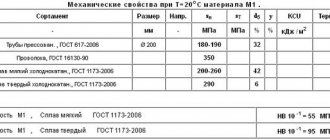
Currently, there are many brands of bronze. They differ in composition, which determines their parameters and scope of application. For convenience, a marking system was created on this basis, including alphabetic and numeric symbols. Thus, alloying additives are designated by the letters first in the name of the chemical elements representing them. The numbers indicate the content of alloy components in fractions of a percent. However, these designations do not contain data on the amount of copper. This value is calculated as the difference between the total composition of bronze and the amount of alloying additives.
Bronze marking makes it easy to determine the grade required for a specific task. To do this, just use special tables. They contain data on the composition, parameters of the alloy and areas of its application.